Exploring SF6's Molecular Structure and Shape

The Complex and Intriguing Nature of Sulfur Hexafluoride

Sulfur hexafluoride, often abbreviated as SF6, is a unique and highly specialized molecule that has found diverse applications across various industries. With its distinctive molecular structure and shape, SF6 presents an intriguing subject for exploration, especially considering its critical role in modern technology and its potential environmental impact.
Molecular Structure: A Six-Fold Symmetry
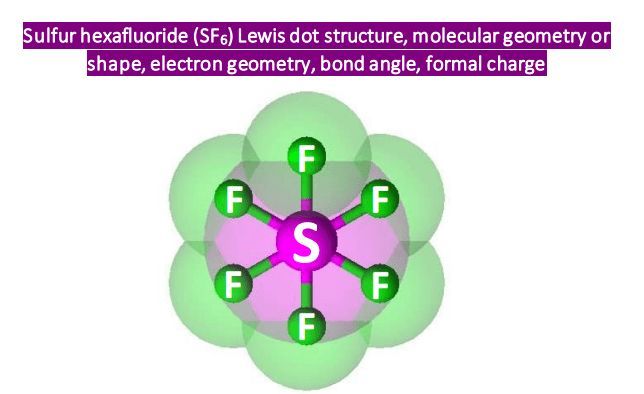
At its core, SF6 boasts a fascinating molecular structure characterized by a central sulfur atom bonded to six fluorine atoms, forming an octahedral geometry. This arrangement, with its high degree of symmetry, is a result of the bonding pattern between the sulfur and fluorine atoms. The sulfur atom, with its six valence electrons, readily forms six covalent bonds with the highly electronegative fluorine atoms, each of which has seven valence electrons.
The resulting molecular structure is highly stable, owing to the complete octets around each atom. This stability is further enhanced by the strong electronegativity difference between sulfur and fluorine, which creates a highly polarized molecule. The sulfur atom, with its larger size, acts as a central hub, while the fluorine atoms, with their smaller size and high electronegativity, form a tightly packed array around the sulfur core.
Shape Determination: From VSEPR Theory to Reality
The shape of SF6 can be determined using the Valence Shell Electron Pair Repulsion (VSEPR) theory, which predicts molecular geometry based on the number of electron pairs surrounding a central atom. In the case of SF6, with six bonding pairs and no lone pairs, the VSEPR theory predicts an octahedral geometry, consistent with the observed molecular structure.
This theory, however, is not without its complexities. The electron repulsion between the fluorine atoms, each carrying a highly negative charge, results in a slight distortion of the ideal octahedral shape. This distortion, though subtle, is a fascinating aspect of SF6’s molecular geometry, highlighting the delicate balance between electronic and steric factors in determining molecular shapes.
Symmetry and Its Implications
The high degree of symmetry in SF6’s molecular structure has significant implications for its physical and chemical properties. The symmetry, combined with the polarizability of the molecule, makes SF6 an excellent dielectric material, with applications in electrical power systems. It is also an efficient insulator, owing to its non-conductive nature, and is used in high-voltage equipment to prevent electrical arcing and enhance safety.
However, this same symmetry and stability also contribute to SF6’s environmental challenges. The molecule’s extreme stability, arising from its symmetric structure, makes it extremely persistent in the atmosphere. SF6 has a global warming potential that is thousands of times higher than that of carbon dioxide, making its release into the atmosphere a significant environmental concern.
Unraveling the Bonding Mysteries
The bonding in SF6 is a complex interplay of ionic and covalent interactions. While the fluorine-sulfur bonds exhibit significant ionic character due to the electronegativity difference, the overall bonding is largely covalent in nature. This complex bonding pattern results in a unique set of physical and chemical properties that distinguish SF6 from other molecules with similar structures.
For instance, the high degree of covalency in SF6’s bonding leads to its high boiling point and low volatility. The molecule’s strong bonds and compact structure make it less susceptible to thermal degradation, contributing to its stability and longevity.
Applications and Beyond
SF6’s unique properties have found numerous applications in diverse fields. Beyond its use in electrical power systems, SF6 is utilized in medical imaging, as a tracer gas in leak detection, and even in the manufacture of flat-panel displays. Its specialized applications highlight the molecule’s versatility and the importance of understanding its molecular structure and shape.
However, with its environmental challenges, the responsible use and containment of SF6 are critical considerations. Ongoing research focuses on developing alternative technologies and materials that can replace SF6 while maintaining its desirable properties.
Conclusion: A Complex Story Unfolds
The exploration of SF6’s molecular structure and shape reveals a complex and fascinating story, showcasing the intricate interplay of bonding, symmetry, and physical properties. From its unique bonding pattern to its environmental implications, SF6 serves as a compelling example of how molecular structure can shape both the applications and challenges of a molecule.
As research continues to unravel the mysteries of SF6 and other complex molecules, the insights gained not only advance our understanding of molecular science but also guide our choices in technology and environmental stewardship.
FAQ Section
What is the primary use of SF6, and why is it so critical in electrical power systems?
+SF6 is primarily used as an insulating and arc-quenching medium in electrical power systems. Its high dielectric strength, combined with its non-conductive nature, makes it ideal for preventing electrical arcing and enhancing safety in high-voltage equipment. Its ability to withstand high electrical stresses without breaking down makes it a critical component in maintaining the reliability and efficiency of power transmission and distribution systems.
How does the molecular structure of SF6 contribute to its environmental challenges?
+The stability of SF6’s molecular structure, resulting from its symmetric octahedral geometry and strong fluorine-sulfur bonds, is a double-edged sword. While this stability makes SF6 an excellent dielectric and insulating material, it also means that the molecule is extremely persistent in the atmosphere. SF6 has a global warming potential that is thousands of times higher than that of carbon dioxide, leading to its classification as a potent greenhouse gas. Its long atmospheric lifetime contributes to its significant environmental impact.
What are some of the ongoing efforts to mitigate the environmental impact of SF6?
+Recognizing the environmental challenges posed by SF6, various initiatives are underway to reduce its release and develop alternatives. These efforts include improving equipment design to minimize leakage, implementing strict handling and recovery procedures, and exploring the use of alternative gases or technologies. Research is also focused on developing more environmentally friendly dielectric materials that can replace SF6 while maintaining its desirable properties.
How does the bonding in SF6 compare to that of other molecules with similar structures?
+The bonding in SF6 is a complex interplay of ionic and covalent interactions. While it shares similarities with other molecules like sulfur tetrafluoride (SF4) and sulfur dioxide (SO2), the presence of six fluorine atoms in SF6 results in a highly stable and symmetric structure. This stability, combined with the polarizability of the molecule, gives SF6 its unique set of properties, setting it apart from other molecules with similar structures.