Understanding the Basics of Ring Closing Metathesis
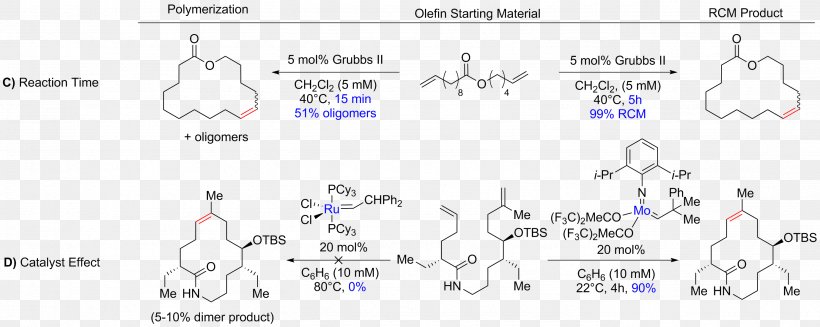
The chemical reaction known as ring-closing metathesis (RCM) is a fundamental process in organic synthesis, offering an elegant approach to creating cyclic compounds. This technique involves the exchange of alkenes, resulting in the formation of new carbon-carbon double bonds and the closure of a ring structure. Delving into the intricacies of RCM reveals a complex yet fascinating mechanism, offering a plethora of opportunities for chemists to design and synthesize diverse molecular architectures.
At its core, ring-closing metathesis relies on the catalytic activity of transition metal complexes, particularly those based on ruthenium or molybdenum. These metal centers facilitate the recombination of alkene fragments, enabling the construction of carbon rings with a range of sizes and complexities. The reaction’s versatility lies in its ability to accommodate various functional groups, making it a powerful tool in synthetic chemistry.
RCM has revolutionized the way we approach organic synthesis, providing a highly efficient and selective method for constructing cyclic compounds. Its impact on drug discovery, material science, and polymer chemistry has been immense, offering new avenues for innovation and problem-solving.
The fundamental concept behind RCM is the metathesis reaction, a process where two molecules exchange fragments to form new products. In the context of RCM, the exchange partners are alkenes, which undergo a series of steps to ultimately form a new carbon ring. This process can be simplified into three main stages: initiation, propagation, and termination.
Initiation: The RCM reaction begins with the activation of a transition metal complex, typically a ruthenium or molybdenum catalyst. This catalyst coordinates with an alkene, initiating the metathesis process.
Propagation: In the propagation phase, the activated catalyst complex interacts with another alkene molecule, resulting in the exchange of fragments and the formation of a new alkene. This newly formed alkene then coordinates with the catalyst, leading to a continuous cycle of alkene exchange and bond formation.
Termination: The reaction concludes when the newly formed alkene has the same structure as the initiating alkene, resulting in a closed ring structure. Alternatively, termination can occur when the catalyst deactivates or when side reactions take place, such as oligomerization or isomerization.
The success of RCM is heavily dependent on the choice of catalyst and reaction conditions. Optimizing these factors can lead to improved reaction efficiency, selectivity, and yield, making it a powerful tool for synthetic chemists.
RCM has found extensive applications across various scientific disciplines. In pharmaceutical research, it has facilitated the synthesis of complex natural products and drug molecules, often with improved efficiency and reduced waste compared to traditional methods. Additionally, RCM has played a crucial role in the development of advanced materials, including polymers with unique properties and nanostructures with tailored functionalities.
The future of RCM looks promising, with ongoing research focused on expanding its scope and improving its applicability. Efforts are directed towards developing new catalysts with enhanced stability and activity, as well as exploring the potential of RCM in emerging fields such as green chemistry and sustainable synthesis.
What is the significance of ring-closing metathesis in organic synthesis?
+RCM provides a highly efficient and selective method for constructing cyclic compounds, offering an alternative to traditional synthesis methods. Its versatility and reliability make it an indispensable tool in organic chemistry, particularly in the synthesis of complex molecules and advanced materials.
How does RCM differ from other metathesis reactions?
+RCM is unique in its ability to close a ring structure, a process not observed in other metathesis reactions. This ring closure mechanism provides access to a wide range of cyclic compounds, making RCM a powerful tool for synthesizing complex molecules with specific ring systems.
What are the challenges associated with RCM, and how can they be overcome?
+One of the main challenges in RCM is the control of selectivity, particularly when dealing with complex substrates. This can be addressed by careful catalyst design, optimizing reaction conditions, and employing strategies such as solvent selection and additive use to influence reaction outcomes.
How has RCM impacted the field of drug discovery and development?
+RCM has revolutionized drug discovery by providing an efficient and selective method for synthesizing complex natural products and drug molecules. Its ability to construct intricate molecular architectures has accelerated the development of novel pharmaceuticals and improved the efficiency of the drug discovery process.
What are some future prospects and research directions in RCM?
+Ongoing research in RCM focuses on developing more stable and active catalysts, as well as exploring its potential in emerging fields like green chemistry and sustainable synthesis. Additionally, efforts are directed towards expanding the scope of RCM to include a wider range of functional groups and reaction conditions.