5 Key Insights About R, the Ideal Gas Constant

R, also known as the universal gas constant or ideal gas constant, is a fundamental constant in chemistry and physics that plays a crucial role in understanding the behavior of gases. It serves as a bridge between the microscopic world of particles and the macroscopic properties of gases, enabling scientists to predict and explain various phenomena. In this article, we delve into five key insights about R, shedding light on its significance and applications.
1. Historical Context and Discovery
The concept of the ideal gas constant has a rich historical background, dating back to the 19th century when scientists were exploring the behavior of gases and developing the kinetic theory of gases. In the early days, researchers such as Robert Boyle and Jacques Charles made groundbreaking observations regarding the relationships between pressure, volume, temperature, and the amount of gas. However, it was not until the work of French physicist Émile Clapeyron and Italian physicist Amedeo Avogadro that the idea of a universal gas constant emerged.
Clapeyron’s research on the relationship between pressure, temperature, and volume in a closed system led to the formulation of the ideal gas law, which incorporated the constant R. Avogadro’s hypothesis, stating that equal volumes of gases, at the same temperature and pressure, contain the same number of molecules, further solidified the need for a constant to account for the ideal gas behavior. Over time, the value of R was refined and standardized, allowing for consistent calculations across various gas-related experiments and theories.
2. The Universal Significance of R
One of the most remarkable aspects of the ideal gas constant is its universality. Regardless of the type of gas or the specific conditions, R remains constant. This constant value, approximately 8.314472 J/(mol·K), is a fundamental parameter that connects the microscopic world of gas molecules with the macroscopic properties observed in experiments. R is a bridge that allows scientists to make predictions and understand the behavior of gases on a larger scale.
For instance, when studying the expansion of gases, scientists can use R to calculate the change in volume or pressure for a given temperature change. Similarly, R plays a crucial role in understanding the relationship between the amount of gas and its pressure, as seen in the ideal gas law: PV = nRT. Here, R acts as a bridge, allowing scientists to quantify the impact of changes in temperature, pressure, and gas amount on the behavior of gases.
3. Applications in Chemistry and Beyond
The ideal gas constant finds extensive applications in various fields, particularly in chemistry and thermodynamics. In chemical reactions involving gases, R is used to calculate the heat exchanged or the change in enthalpy, providing insights into the energy changes that occur during a reaction. This is especially crucial in understanding and predicting the behavior of gases in chemical processes, such as combustion or gas-phase reactions.
Moreover, R is not limited to chemistry alone. It plays a significant role in physics, particularly in the study of thermodynamic systems and the behavior of gases in different states. For example, in the study of atmospheric physics, R is used to understand the behavior of gases in the Earth’s atmosphere, helping scientists predict weather patterns and climate phenomena. Additionally, R is essential in the design and operation of various gas-based technologies, such as engines, refrigeration systems, and gas storage facilities.
4. Experimental Verification and Precision
The accuracy and precision of the ideal gas constant are of utmost importance in scientific research. Experimental verification of R’s value is essential to ensure the reliability of calculations and predictions. Over the years, scientists have conducted numerous experiments to determine R with increasing accuracy. One of the most precise methods involves measuring the speed of sound in a gas and using the ideal gas law to calculate R.
The precision of R’s value has significant implications for scientific research. Even slight variations in R can lead to significant discrepancies in calculations, especially when dealing with large-scale phenomena or delicate processes. Therefore, ongoing efforts to refine and standardize R’s value are crucial for maintaining the integrity of scientific investigations involving gases.
5. R’s Role in Understanding Gas Behavior
R is an indispensable tool for understanding and predicting the behavior of gases under various conditions. It allows scientists to make quantitative assessments of gas properties, such as pressure, volume, and temperature, and their interrelationships. By incorporating R into equations, scientists can model and simulate gas behavior, aiding in the design of efficient systems and processes.
For example, in the study of gas dynamics, R is used to calculate the adiabatic index, which is crucial for understanding the behavior of gases in turbines, engines, and other fluid systems. Additionally, R is essential in the analysis of gas mixtures, helping scientists determine the composition and properties of gases in complex environments, such as the Earth’s atmosphere or industrial gas mixtures.
In conclusion, the ideal gas constant, R, is a fundamental constant that serves as a bridge between the microscopic and macroscopic worlds of gases. Its universality, applications, and precision make it an indispensable tool in scientific research and technological advancements. By understanding the insights presented in this article, we gain a deeper appreciation for the role of R in our understanding of gases and its contributions to various fields.
FAQ Section
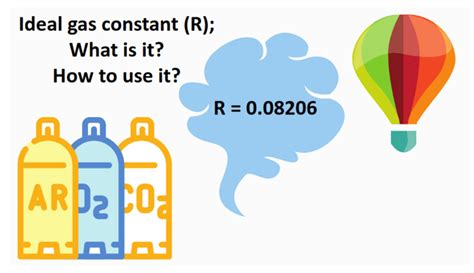
What is the ideal gas constant used for?
+The ideal gas constant, R, is used to relate the pressure, volume, temperature, and amount of an ideal gas. It plays a crucial role in various gas-related calculations, including the ideal gas law (PV = nRT), allowing scientists to predict and understand the behavior of gases under different conditions.
<div class="faq-item">
<div class="faq-question">
<h3>How accurate is the value of R in scientific research?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>The accuracy of R's value is of utmost importance in scientific research. Ongoing efforts to refine and standardize R's value ensure the reliability of calculations and predictions. Currently, the accepted value of R is approximately 8.314472 J/(mol·K), which has been determined through precise experimental methods.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What are some practical applications of R in everyday life?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>R finds applications in various everyday scenarios. For instance, in weather forecasting, R is used to understand atmospheric gas behavior, helping meteorologists predict weather patterns. Additionally, R is essential in the design of efficient engines, refrigeration systems, and even the operation of gas-based home appliances.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>How does R relate to other gas constants, such as the Boltzmann constant (k)?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>The ideal gas constant, R, is related to the Boltzmann constant, k, through the gas constant, R = NA k, where NA is Avogadro's constant. While R is used to describe the behavior of gases as a whole, k is used to describe the behavior of individual gas particles and their kinetic energy.</p>
</div>
</div>
</div>