Periodic Table Group Numbers: Unveiled!
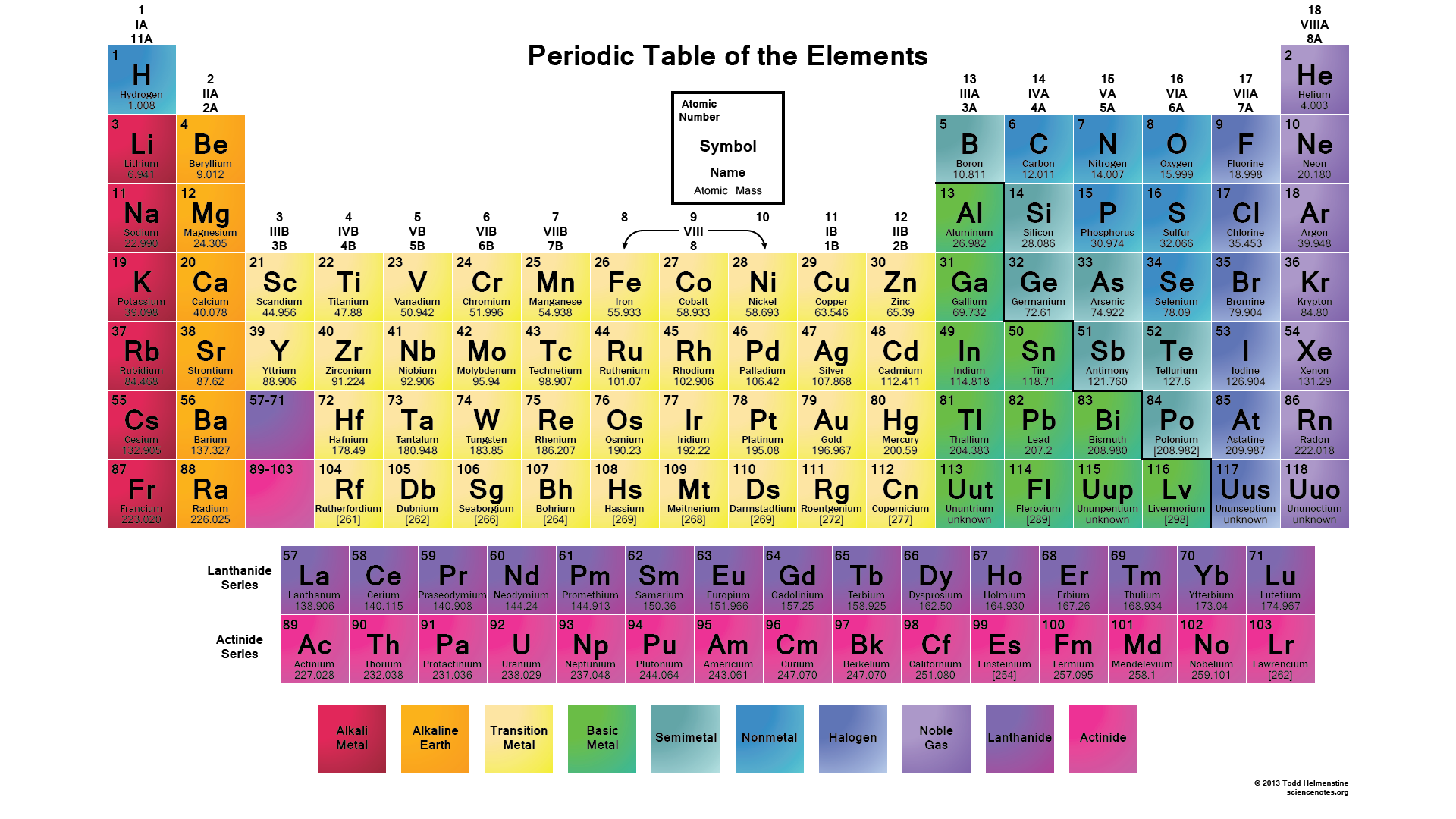
Unraveling the Periodic Table’s Group Numbers

At first glance, the periodic table may appear as a simple arrangement of elements, but a closer inspection reveals a sophisticated system of organization. The vertical columns, known as groups or families, provide a unique classification system, with each group sharing common properties and trends. The group numbers, a vital part of this system, offer a concise way to identify and understand these elemental relationships.
Historical Perspective: The Evolution of Group Numbers

The concept of grouping elements is not new, but the precise method and terminology have evolved over time. Early chemists, such as Mendeleev, recognized the importance of grouping elements based on their properties, but the modern system of group numbers is a product of more recent developments.
Understanding Group Numbers: A Practical Guide
So, how do these group numbers actually work? Let’s explore some practical examples and insights:
The Main-Group Elements: These are the familiar elements of everyday life, often referred to as the ‘representative elements.’ They include groups 1-2 (alkali and alkaline earth metals) and groups 13-18 (boron group, carbon group, nitrogen group, oxygen group, and halogens).
The Transition Metals: These elements, occupying the middle portion of the periodic table, have unique properties due to their incomplete d-orbital electron configurations.
The Inner Transition Metals: Also known as the lanthanides and actinides, these groups are less familiar but no less important.
Real-World Applications of Group Numbers
The practical applications of group numbers are far-reaching and impact various fields:
Chemistry and Materials Science: Understanding group behavior is essential for predicting chemical reactions and designing new materials with specific properties.
Environmental Science: Group numbers help identify elements that may be harmful or beneficial to the environment, aiding in pollution control and ecological research.
Medicine and Biology: Knowledge of group behavior is crucial for understanding the role of elements in biological systems and developing medical treatments.
Industry and Technology: From the development of new alloys to the design of high-tech electronics, group numbers play a vital role in modern industry.
Future Trends and Emerging Research

As our understanding of the periodic table continues to evolve, so too does our knowledge of group numbers. Ongoing research focuses on:
- Exploring New Materials: Scientists are investigating the unique properties of elements in less-studied groups, particularly the rare earths and actinides, for potential use in technology and energy applications.
- Refining Group Definitions: While the basic group system is established, ongoing research aims to refine and clarify the boundaries and properties of each group, especially in the transition metals.
- Educational Initiatives: Efforts are underway to improve the teaching of group numbers and the periodic table, ensuring that students understand these fundamental concepts.
Conclusion: The Power of Group Numbers
In conclusion, the group numbers of the periodic table offer a powerful tool for understanding the vast array of elements that make up our world. From the familiar alkali metals to the less-known lanthanides, each group has its unique story and role to play. As we continue to explore and understand these elements, the group numbers will remain a vital part of our scientific toolkit.
What are the main groups in the periodic table and their key characteristics?
+The main groups in the periodic table are the alkali metals (Group 1), alkaline earth metals (Group 2), boron group (Group 13), carbon group (Group 14), nitrogen group (Group 15), oxygen group (Group 16), and halogens (Group 17). Each group has distinct properties and behaviors, with common trends in reactivity and electron configurations.
How do group numbers help in predicting chemical reactions and properties?
+Group numbers provide a quick way to identify elements with similar chemical behaviors. Elements within the same group often have the same number of valence electrons, leading to similar reactivity and physical properties. This predictability is a powerful tool for chemists and scientists.
Are there any exceptions to the trends within groups?
+Yes, while groups generally follow consistent patterns, there are exceptions. This is particularly true in the transition metals and inner transition metals, where the incomplete filling of d and f orbitals can lead to unique and sometimes unpredictable behaviors.
How have group numbers evolved over time in the periodic table?
+The concept of grouping elements based on properties has been around since the 19th century, but the modern system of group numbers was standardized in the 1940s by IUPAC. This system has since been refined, especially with the discovery and understanding of the unique properties of the rare earth and actinide groups.
What are some real-world applications of understanding group numbers in the periodic table?
+Understanding group numbers has applications in various fields. In chemistry and materials science, it aids in predicting chemical reactions and designing new materials. In environmental science, it helps identify elements that may impact ecosystems. In medicine and biology, it’s crucial for understanding the role of elements in biological systems. In industry and technology, group numbers are vital for developing new products and processes.



