Unraveling the Mystery: First-Order Rate Laws
Diving into the world of chemical kinetics, we often encounter the enigmatic concept of first-order rate laws. These laws govern the intricate dance of molecules during chemical reactions, yet their mysteries remain partially unveiled. Today, we embark on a journey to unravel the secrets behind these fundamental principles, shedding light on their significance and practical applications.
The Essence of First-Order Rate Laws

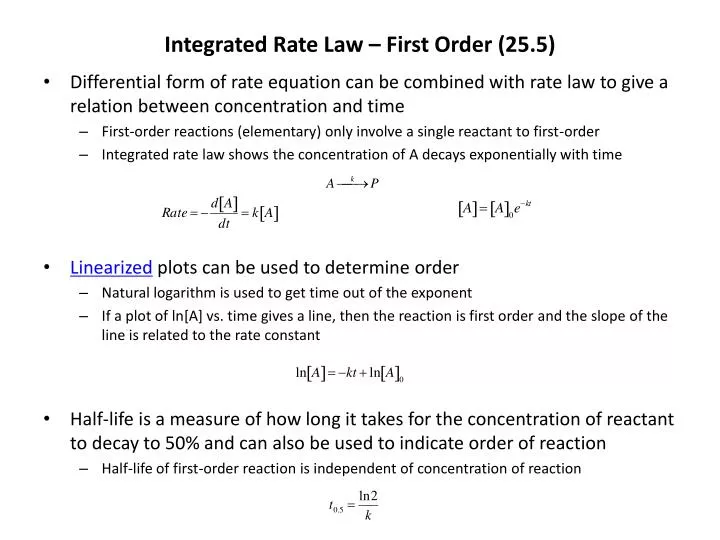
First-order rate laws describe the relationship between the rate of a chemical reaction and the concentration of one of its reactants. It is a fundamental concept in chemical kinetics, providing insights into how reactions proceed and offering a predictive tool for chemists and researchers.
The mathematical representation of a first-order rate law is simple yet powerful:
Here, ‘Rate’ represents the speed at which the reaction occurs, ‘k’ is the rate constant, and ‘[A]’ signifies the concentration of the reactant in the reaction. This equation tells us that the rate of the reaction is directly proportional to the concentration of the reactant ‘A’.
However, this simplicity belies the complexity and intrigue that surround first-order rate laws.
Unveiling the Enigmas
The Rate Constant (k)
The rate constant, often denoted by ‘k’, is a pivotal element in first-order rate laws. It represents the intrinsic reactivity of the reactant and is influenced by various factors such as temperature, pressure, and the nature of the reaction.
Determining the rate constant involves intricate experimental procedures and meticulous analysis. Researchers employ techniques like spectrophotometry, chromatography, and mass spectrometry to measure the rate of reaction at different concentrations of the reactant.
Advantage: Predictability
Once determined, the rate constant allows chemists to predict the rate of reaction under various conditions, making it a powerful tool for process optimization and reaction design.
<div class="con">
<h3>Challenge: Temperature Dependence</h3>
<p>Rate constants are highly temperature-dependent, which means they may vary significantly across different experimental conditions. This variability poses a challenge in extrapolating results to real-world scenarios.</p>
</div>
Concentration Dependence
First-order rate laws highlight the critical role of reactant concentration in determining the reaction rate. This relationship is particularly significant in reactions where one reactant is present in significantly lower concentrations than the others.
- In a first-order reaction, doubling the concentration of the reactant 'A' will double the reaction rate.
- Conversely, if the concentration of 'A' is halved, the rate will also decrease by half.
- This linear relationship between concentration and rate is a hallmark of first-order kinetics and provides a straightforward means of controlling reaction rates.
Temperature Effects
Temperature is a critical factor influencing the rate constant and, by extension, the reaction rate. As temperature increases, molecules gain more kinetic energy, leading to more frequent and energetic collisions. This, in turn, accelerates the reaction rate.
Here, ‘A’ is the pre-exponential factor, ‘Ea’ is the activation energy, ‘R’ is the gas constant, and ’T’ is the temperature in Kelvin. This equation shows how temperature (’T’) exponentially affects the rate constant (‘k’).
Real-World Applications
First-order rate laws find extensive application in various fields, including:
- Pharmaceutical Industry: Understanding reaction kinetics is crucial for drug development, as it enables the optimization of synthesis processes and the prediction of drug stability.
- Environmental Science: First-order kinetics models are used to study the breakdown of pollutants in the environment, aiding in the development of effective remediation strategies.
- Materials Science: Researchers utilize first-order kinetics to investigate the aging and degradation of materials, which is critical for the development of durable and sustainable materials.
Conclusion
The mysteries of first-order rate laws, though partially unveiled, continue to captivate and challenge researchers. Their fundamental role in chemical kinetics and their wide-ranging applications make them an essential topic for anyone studying chemistry or involved in chemical processes.
By delving into the intricacies of first-order rate laws, we not only enhance our understanding of chemical reactions but also equip ourselves with valuable tools for practical applications across diverse fields.
How do first-order rate laws differ from higher-order rate laws?
+First-order rate laws describe reactions where the rate depends solely on the concentration of one reactant. In contrast, higher-order rate laws, such as second-order or third-order, involve multiple reactants, with the rate depending on the concentrations of two or more reactants, respectively.
<div class="faq-item">
<div class="faq-question">
<h3>Can first-order rate laws be applied to all chemical reactions?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>No, first-order rate laws are specific to reactions where the concentration of one reactant is the dominant factor affecting the reaction rate. Not all reactions follow first-order kinetics, and understanding the reaction mechanism is crucial in determining the appropriate rate law.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>How do researchers determine the rate constant (k) for a first-order reaction?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Researchers typically employ experimental methods, such as measuring the rate of reaction at different reactant concentrations and fitting the data to a first-order rate law equation. This process involves a combination of laboratory experiments and mathematical modeling.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What are some practical implications of understanding first-order rate laws in the pharmaceutical industry?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Understanding first-order rate laws allows pharmaceutical researchers to optimize drug synthesis processes, predict drug stability, and design controlled-release formulations. This knowledge is crucial for ensuring the efficacy and safety of medications.</p>
</div>
</div>
</div>