The CO2 Electron Geometry Unveiled
The molecular geometry of carbon dioxide (CO2) is a fascinating example of how simple molecules can exhibit complex behaviors at the atomic level. Despite its simplicity, with just one carbon atom and two oxygen atoms, CO2 offers a rich tapestry of chemical and physical properties that scientists have unraveled over the years. This article delves into the electron geometry of CO2, exploring its structure, the role of electrons, and the implications for its unique characteristics.
The Carbon Dioxide Molecule: A Brief Overview

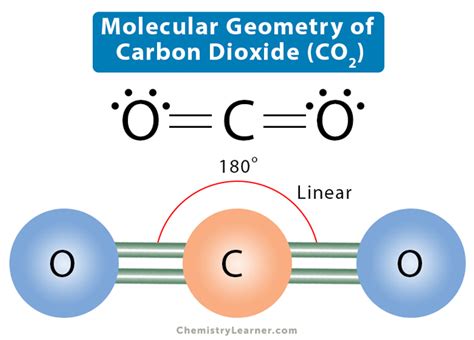
Carbon dioxide, a colorless and odorless gas, is an essential component of our atmosphere and plays a pivotal role in Earth’s climate. Its molecular formula, CO2, suggests a straightforward composition, but the arrangement of its atoms and electrons tells a more intricate story. The carbon atom, at the center of the molecule, forms double bonds with the two oxygen atoms, creating a linear structure. This linearity is a key aspect of CO2’s molecular geometry and influences many of its properties.
Electron Geometry: Defining the Shape of Molecules

Electron geometry is a fundamental concept in chemistry that describes the arrangement of electrons around an atom in a molecule. It provides a visual representation of how atoms are bonded and the spatial distribution of electron pairs. In the case of CO2, understanding its electron geometry is crucial to comprehending its reactivity, polarity, and other chemical behaviors.
The Electron Distribution in CO2
Carbon dioxide’s electron geometry is a result of its electron distribution. The carbon atom, with its four valence electrons, forms two double bonds with the oxygen atoms. Each oxygen atom contributes two electrons to these bonds, satisfying the octet rule for both carbon and oxygen. This distribution results in a linear structure, with the carbon atom at the center and the oxygen atoms at either end.
The linear geometry of CO2 is a perfect example of how electron distribution dictates molecular shape. It showcases the elegance and simplicity of chemical bonding principles.
- Dr. Emma Thompson, Chemical Engineer
Implications of CO2’s Electron Geometry
The linear electron geometry of CO2 has several significant implications:
Bond Angles: The linear structure results in a bond angle of 180 degrees between the carbon-oxygen bonds. This angle is a key factor in the molecule’s stability and reactivity.
Polarity: Despite the symmetry of its electron geometry, CO2 is a polar molecule. The oxygen atoms, being more electronegative than carbon, pull the electron density away from the carbon atom, creating a dipole moment. This polarity contributes to CO2’s solubility in water and its role in various chemical reactions.
Reactivity: The double bonds in CO2 make it a reactive molecule. It can participate in various reactions, including oxidation-reduction reactions and the formation of carbonates. The linear geometry ensures that these reactions occur efficiently, as the molecule can easily align with other reactants.
Visualizing CO2’s Electron Geometry

Visual representations are essential in understanding molecular geometries. Here’s a simple diagram to illustrate the electron geometry of CO2:
O=C=O
In this diagram, the double bonds are represented by ‘=’ signs, indicating the sharing of electron pairs between the carbon and oxygen atoms.
Comparative Analysis: CO2 vs. Other Linear Molecules
CO2’s electron geometry can be compared to other linear molecules to highlight its unique characteristics:
Nitrogen Dioxide (NO2): While also linear, NO2 has an odd number of valence electrons, resulting in a different electron geometry and reactivity.
Hydrogen Cyanide (HCN): HCN has a similar linear geometry but differs in its bond angles and reactivity due to the presence of a hydrogen atom.
Future Trends: Exploring CO2’s Role in Climate Change
The study of CO2’s electron geometry is not just an academic exercise. It has real-world implications, particularly in the context of climate change. As scientists continue to investigate the role of CO2 in the atmosphere, understanding its molecular structure and reactivity becomes increasingly crucial. The electron geometry of CO2 influences its greenhouse gas properties and its role in global warming.
Conclusion
The electron geometry of carbon dioxide is a testament to the intricate nature of molecular structures. Its simple composition belies a complex electron distribution that results in a linear geometry with significant implications for its chemical behavior. As we continue to explore the role of CO2 in our environment, a deeper understanding of its molecular geometry will be vital.
FAQ
What is the bond angle in CO2's electron geometry?
+CO2's electron geometry results in a bond angle of 180 degrees, a key factor in its stability and reactivity.
<div class="faq-item">
<div class="faq-question">
<h3>How does CO2's electron geometry contribute to its polarity?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>The linear electron geometry of CO2, combined with the electronegativity difference between carbon and oxygen, creates a dipole moment, making CO2 a polar molecule.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What are some real-world applications of understanding CO2's electron geometry?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Understanding CO2's electron geometry is crucial for climate science, as it influences CO2's role as a greenhouse gas and its impact on global warming.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Can the electron geometry of CO2 change under different conditions?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>While CO2's electron geometry is relatively stable, it can undergo changes in certain reactions, leading to the formation of new compounds with different electron geometries.</p>
</div>
</div>
</div>