Unveiling the Metalloids: Their Periodic Table Homes

Metalloids, a unique group of elements, often spark curiosity due to their enigmatic nature. These elements, with characteristics that bridge the gap between metals and non-metals, have fascinating stories and important roles in various industries. Let’s embark on a journey to explore their homes on the periodic table, uncovering their secrets and significance.
The Metalloids’ Enigma
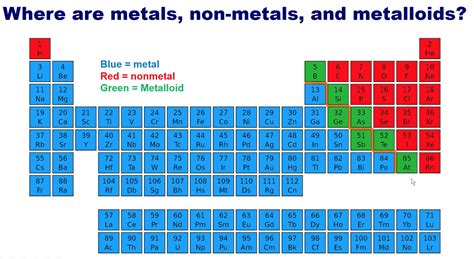
In the vast landscape of the periodic table, metalloids stand out as enigmatic figures. They possess traits that defy easy categorization, showcasing properties of both metals and non-metals. This duality makes them intriguing subjects of study for scientists and enthusiasts alike.
Boron: The Versatile Innovator
Boron, the first metalloid we encounter, is a master of versatility. With its atomic number 5, it forms the foundation of the metalloid family. Known for its hardness and high melting point, boron is a key player in various industries. From its use in high-strength ceramics to its role in advanced electronics, boron’s impact is far-reaching.
Applications:
- Ceramics and Glass: Boron compounds enhance the strength and durability of ceramics, making them ideal for extreme environments.
- Nuclear Industry: Boron’s neutron-absorbing properties make it crucial in nuclear reactors for controlling nuclear reactions.
- Semiconductors: Boron is a key dopant in semiconductor manufacturing, enabling the creation of advanced electronic devices.
Silicon: The Silicon Age Element
Silicon, with its atomic number 14, is a true star in the metalloid constellation. It is the second most abundant element in the Earth’s crust, and its impact on modern life is undeniable. Silicon’s unique electrical properties have revolutionized the digital world.
Silicon’s Impact:
- Semiconductors: Silicon is the backbone of the semiconductor industry, powering computers, smartphones, and countless electronic devices.
- Solar Energy: Silicon-based solar cells have driven the growth of renewable energy, offering a sustainable alternative.
- Biomedical: Silicon’s biocompatibility makes it valuable in medical implants and drug delivery systems.
Germanium: The Historic Metalloid
Germanium, atomic number 32, has a rich history. Discovered in the late 19th century, it played a pivotal role in the early days of electronics. Its semiconductor properties made it a key material in the development of transistors, shaping the modern electronic era.
Historical Significance:
- Transistor Revolution: Germanium transistors replaced bulky vacuum tubes, leading to compact and efficient electronic devices.
- Radar Technology: During World War II, germanium diodes played a crucial role in radar systems, aiding in military operations.
- Fiber Optics: Germanium-based fibers have revolutionized telecommunications, enabling high-speed data transmission.
Arsenic: The Toxic Metalloid
Arsenic, atomic number 33, is a metalloid with a dark side. While it shares properties with other metalloids, its toxicity sets it apart. Arsenic compounds have been used throughout history, from ancient poisons to modern industrial applications.
Arsenic’s Complex Nature:
- Toxicity: Arsenic is highly toxic, and its compounds have been used as poisons and insecticides.
- Medicine: Paradoxically, arsenic has medicinal applications, especially in treating certain types of cancer.
- Environmental Concerns: Arsenic contamination in water and soil is a global concern, requiring careful management.
Antimony: The Shining Metalloid
Antimony, atomic number 51, is a metalloid with a unique shine. Its lustrous appearance and diverse properties make it valuable in various industries. Antimony is often associated with strength and durability.
Antimony’s Applications:
- Alloys: Antimony is added to lead alloys, improving their strength and durability, especially in battery grids.
- Flame Retardants: Antimony compounds are used as flame retardants in plastics and textiles, enhancing fire safety.
- Catalysts: In chemical reactions, antimony-based catalysts play a crucial role in accelerating processes.
Tellurium: The Rare Metalloid
Tellurium, atomic number 52, is a rare metalloid with intriguing properties. Its scarcity adds to its allure, and its applications are diverse and innovative. Tellurium’s unique electrical and optical properties make it a subject of ongoing research.
Tellurium’s Innovations:
- Solar Cells: Tellurium-based solar cells offer high efficiency, providing an alternative to silicon-based cells.
- Thermoelectric Devices: Tellurium’s ability to convert heat into electricity makes it valuable in waste heat recovery systems.
- Data Storage: Tellurium-based materials are explored for next-generation data storage technologies.
Metalloids in the Modern World
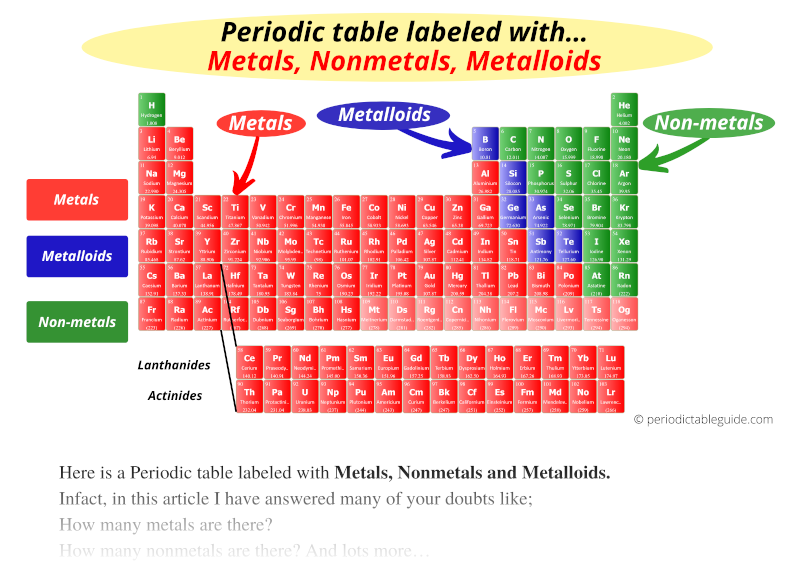
In today’s world, metalloids play an indispensable role. From the digital revolution driven by silicon to the innovative applications of tellurium, their impact is profound. These elements, with their unique properties, have shaped industries and continue to inspire scientific advancements.
The Future of Metalloids:
- Materials Science: Ongoing research aims to harness the potential of metalloids in creating advanced materials with unique properties.
- Energy Transition: Metalloids are key players in the transition to sustainable energy, from solar cells to battery technologies.
- Healthcare: Metalloids offer promising applications in medical imaging, diagnostics, and targeted therapies.
Exploring Further
The world of metalloids is vast and fascinating, offering countless avenues for exploration. As we delve deeper into their properties and applications, we uncover a wealth of knowledge and potential.
What makes metalloids unique compared to metals and non-metals?
+Metalloids possess a blend of properties from both metals and non-metals. They often exhibit intermediate conductivity, falling between good conductors like metals and insulators like non-metals. Additionally, their melting points and boiling points are typically lower than metals but higher than non-metals.
How are metalloids used in everyday life?
+Metalloids have a wide range of everyday applications. Silicon, for instance, is ubiquitous in electronic devices. Antimony is used in batteries, and tellurium has applications in solar energy and data storage. These elements enhance our daily lives through technology and energy solutions.
Are there any environmental concerns associated with metalloids?
+Yes, some metalloids like arsenic pose environmental challenges due to their toxicity. Proper management and responsible use are crucial to mitigate potential risks. Sustainable practices and recycling efforts can help address these concerns.
What are some emerging applications of metalloids in technology?
+Metalloids are at the forefront of technological advancements. Tellurium, for example, is being explored for high-capacity data storage. Germanium is crucial in advanced optics, and silicon continues to drive innovations in electronics and renewable energy.
As we unravel the mysteries of the metalloids, their importance in our modern world becomes increasingly clear. These elements, with their unique characteristics, have carved out significant roles in various industries, shaping our lives in ways we often take for granted. The periodic table’s metalloid section is a treasure trove of fascinating stories and untapped potential, waiting to be explored further.


