3 Ways to Determine Freezing Point
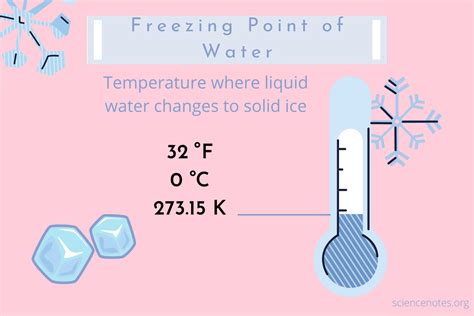
The freezing point of a substance is a critical parameter in various scientific, industrial, and everyday applications. It signifies the temperature at which a liquid transforms into a solid state, and accurately determining this point is essential for quality control, safety, and process optimization. Here, we explore three distinct methods to ascertain the freezing point of a substance, each with its own advantages and considerations.
Classical Cooling Curve Method: This traditional approach involves gradually lowering the temperature of a sample while monitoring its behavior. Here’s a step-by-step breakdown:
- Preparation: Begin by thoroughly cleaning and calibrating the necessary equipment, including a temperature-controlled chamber, a thermometer, and a sample container.
- Sample Selection: Choose a representative sample of the substance, ensuring it is free from impurities and of a consistent composition.
- Temperature Control: Slowly decrease the temperature within the chamber, maintaining a steady and controlled rate of cooling. This gradual approach helps prevent sudden phase changes that might obscure the freezing point.
- Observation: Continuously monitor the sample’s temperature and physical state. Look for subtle changes, such as a decrease in viscosity or the appearance of solid crystals, which may indicate the onset of freezing.
- Data Recording: Note the temperature readings at regular intervals, creating a detailed temperature-time profile, known as a cooling curve.
- Analysis: Once the sample has solidified, analyze the cooling curve to identify the precise temperature at which the transition from liquid to solid occurred. This temperature is the freezing point of the substance.
Differential Scanning Calorimetry (DSC): DSC is a highly sensitive technique that measures the heat flow associated with phase transitions. Here’s how it works:
- Sample Preparation: Prepare a small, uniform sample of the substance, ensuring it is compatible with the DSC equipment.
- Instrument Setup: Calibrate the DSC instrument, which typically consists of a sample cell and a reference cell. Both cells are heated or cooled at the same rate while the instrument measures the heat flow difference between the two.
- Temperature Program: Design a temperature profile that gradually increases or decreases the temperature, depending on the expected freezing point range of the substance.
- Data Collection: During the temperature program, the DSC instrument continuously records the heat flow difference between the sample and reference cells.
- Data Analysis: After the experiment, analyze the DSC thermogram, which plots heat flow against temperature. The freezing point is identified by a sharp endothermic peak, indicating the absorption of heat as the substance transitions from liquid to solid.
Optical Microscopy: This visual approach provides a unique perspective on the freezing process:
- Sample Mounting: Prepare a small volume of the substance on a transparent microscope slide, ensuring it is thin enough to allow light to pass through.
- Microscope Setup: Use a polarized light microscope equipped with a temperature-controlled stage. This setup allows for the observation of the sample at various temperatures.
- Temperature Control: Slowly decrease the temperature of the stage, creating a controlled cooling environment for the sample.
- Visual Observation: Observe the sample through the microscope, looking for changes in the appearance of the liquid. As the temperature drops, the liquid may start to exhibit signs of crystallization or the formation of solid particles.
- Image Capture: Capture images or videos of the sample at different temperature points, creating a visual record of the freezing process.
- Analysis: Review the captured images or videos to pinpoint the temperature at which the first signs of solidification occur. This temperature is the freezing point of the substance.
Each of these methods offers a unique perspective on determining the freezing point, catering to different experimental requirements and equipment availability. By understanding these approaches, researchers and scientists can choose the most suitable technique for their specific needs, ensuring accurate and reliable results in their freezing point determinations.
Pros of Classical Cooling Curve Method
- Simple and accessible equipment requirements.
- Provides a visual understanding of the freezing process.
- Allows for the observation of multiple phase transitions.
Cons of Classical Cooling Curve Method
- Slower and less sensitive than other methods.
- Requires careful observation and interpretation of data.
- May not be suitable for substances with rapid or complex freezing behavior.
Pros of Differential Scanning Calorimetry (DSC)
- Highly sensitive and accurate.
- Can analyze a wide range of substances.
- Provides quantitative data on heat flow during freezing.
Cons of Differential Scanning Calorimetry (DSC)
- Requires specialized and expensive equipment.
- May not be suitable for large or opaque samples.
- Interpretation of data may require advanced training.
Pros of Optical Microscopy
- Provides a direct visual observation of freezing.
- Can capture dynamic changes during the freezing process.
- Suitable for small, transparent samples.
Cons of Optical Microscopy
- Limited to transparent samples.
- May require specialized microscopy equipment.
- Interpretation of visual data can be subjective.
What are some common challenges in determining freezing points accurately?
+Accurate freezing point determination can be challenging due to factors like impurities, the presence of multiple components, and the potential for supercooling or superheating. Impurities can alter the freezing behavior, while multiple components may exhibit complex phase transitions. Additionally, substances can supercool or superheat, delaying or preventing the onset of freezing.
Can these methods be used for substances with complex freezing behaviors?
+Yes, all three methods can provide insights into complex freezing behaviors. The Classical Cooling Curve Method offers a visual understanding, DSC provides quantitative data, and Optical Microscopy allows for dynamic observation. However, for extremely complex substances, additional techniques like X-ray diffraction or Raman spectroscopy might be required.
Are there any safety considerations when determining freezing points?
+Absolutely. Safety is paramount when working with substances, especially at extreme temperatures. Ensure proper personal protective equipment (PPE) and follow laboratory safety guidelines. Be cautious with substances that might be hazardous, reactive, or toxic, and always work within the capabilities of your equipment and expertise.
How can I choose the most suitable method for my specific substance and experimental goals?
+The choice of method depends on factors such as the nature of the substance, the required level of accuracy, available equipment, and the specific experimental objectives. Consider the advantages and limitations of each method outlined above, and consult with experienced researchers or specialists in your field for guidance.



