Aluminum's Valence Electrons: A Quick Guide
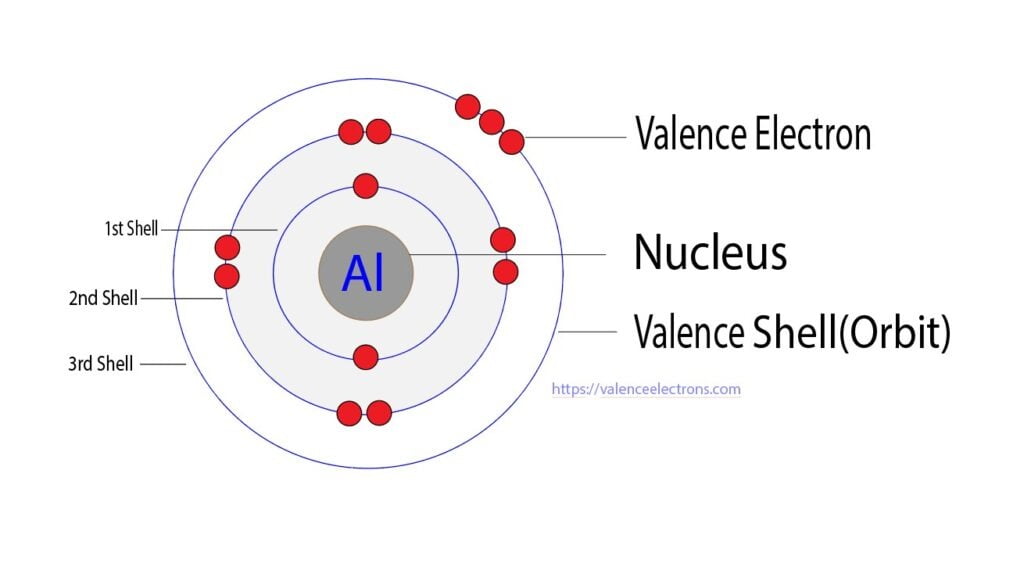
Aluminum, with its symbol Al and atomic number 13, is a fascinating element that plays a crucial role in various industries and everyday life. While it might seem like a simple metal, its electron configuration and, more specifically, its valence electrons, hold the key to understanding its unique properties and behaviors. Let’s dive into the world of aluminum’s valence electrons and uncover the secrets they reveal.
Electron configuration is the arrangement of electrons in an atom's energy levels, providing a blueprint for its behavior.
Electron Configuration 101
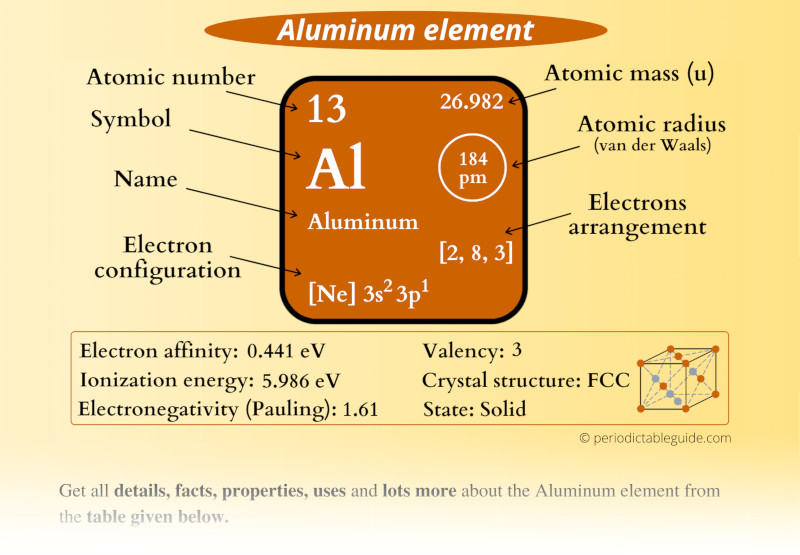
At the heart of understanding aluminum’s valence electrons is the concept of electron configuration. This fundamental principle in chemistry dictates how electrons are distributed among an atom’s energy levels or shells. For aluminum, this configuration is a precise arrangement that sets it apart from other elements.
The first shell, closest to the nucleus, can hold a maximum of two electrons. The second shell, a bit further out, can accommodate up to eight electrons. However, it’s the third shell, the outermost one for aluminum, that’s of particular interest when discussing valence electrons. This outer shell is known as the valence shell, and it’s here that aluminum’s unique characteristics become apparent.
The Role of Valence Electrons

Valence electrons are the electrons found in an atom’s outermost energy level or valence shell. These electrons are the ones involved in chemical bonding and reactions, making them crucial for understanding an element’s reactivity and behavior. In the case of aluminum, its valence electrons play a significant role in its ability to form compounds and interact with other elements.
Pros of Aluminum's Valence Electrons
- High reactivity: Aluminum's three valence electrons make it highly reactive, enabling it to form a wide range of compounds.
- Versatility: This reactivity translates to versatility, as aluminum can be used in various industries, from construction to aerospace.
- Corrosion resistance: When aluminum forms a protective oxide layer, it resists corrosion, making it ideal for outdoor applications.
Cons of Aluminum's Valence Electrons
- Limited stability: With only three valence electrons, aluminum tends to be less stable than elements with complete outer shells.
- Oxidation concerns: While the oxide layer provides protection, it can also cause issues in certain environments, leading to potential corrosion.
- Electrical conductivity: Aluminum's valence electrons make it a good conductor of electricity, but this property can be a drawback in some applications.
Aluminum’s Valence Electron Configuration
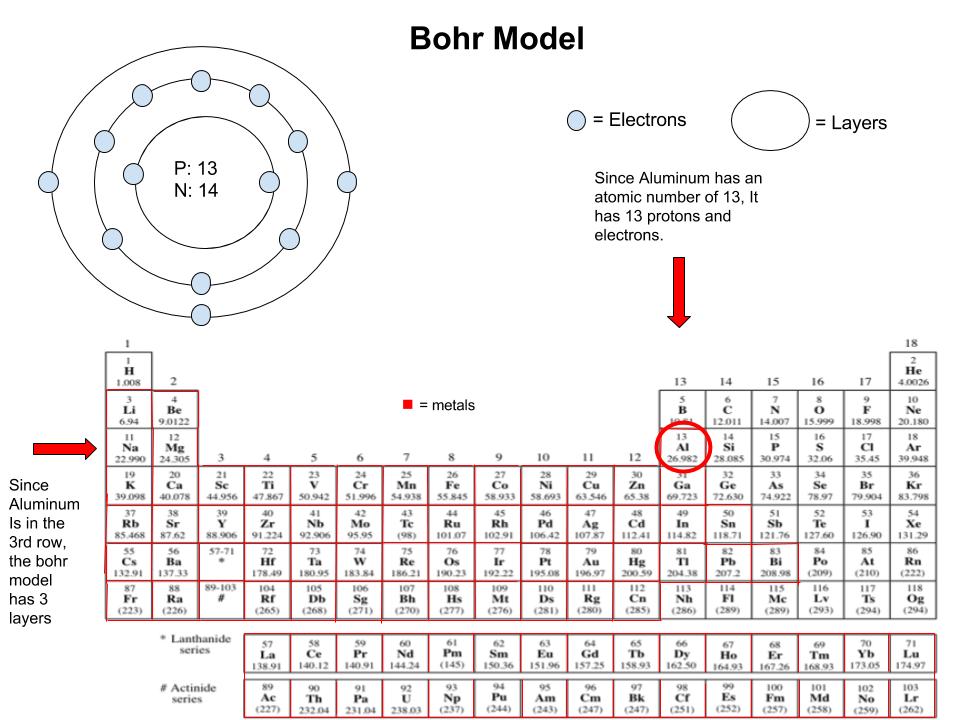
Now, let’s take a closer look at aluminum’s valence electron configuration. With its atomic number of 13, aluminum has a total of 13 electrons distributed across its energy levels. The first two shells are filled, with two electrons in the first shell and eight in the second. However, it’s the third shell that holds the key to aluminum’s valence electrons.
The third shell of aluminum contains three electrons, making it an incomplete shell. This incomplete shell is what gives aluminum its unique properties and reactivity. With only three valence electrons, aluminum is eager to participate in chemical reactions, seeking to achieve a more stable configuration by either losing or gaining electrons.
Chemical Behavior of Aluminum
The presence of three valence electrons in aluminum’s outermost shell influences its chemical behavior in several ways:
- Bonding: Aluminum readily forms ionic bonds with other elements, especially those with a higher electronegativity. This bonding behavior allows aluminum to create a wide array of compounds, contributing to its versatility in various industries.
- Oxidation: One of the most well-known properties of aluminum is its ability to form a protective oxide layer when exposed to oxygen. This oxide layer, composed of aluminum oxide (Al2O3), provides corrosion resistance, making aluminum a popular choice for outdoor applications.
- Reactivity: With its three valence electrons, aluminum is highly reactive. It readily reacts with various substances, including acids and bases, leading to the formation of different compounds. This reactivity is both a strength and a challenge, depending on the application.
Aluminum’s Applications and Benefits
The unique properties arising from aluminum’s valence electron configuration have led to its widespread use in numerous industries:
- Construction: Aluminum’s lightweight nature, combined with its strength and corrosion resistance, makes it an ideal material for construction. It’s used in everything from building frames to roofing materials.
- Transportation: In the transportation industry, aluminum’s low density and high strength-to-weight ratio make it a preferred choice for vehicle manufacturing. It’s used in cars, trains, and even aircraft, contributing to fuel efficiency and performance.
- Packaging: Aluminum’s excellent barrier properties and corrosion resistance make it a popular choice for packaging, especially for food and beverages. Aluminum cans and foil are commonly used to preserve products.
- Electrical Conductivity: Despite being a drawback in some cases, aluminum’s electrical conductivity makes it suitable for electrical transmission lines and wiring. Its lightweight nature reduces the structural demands on support systems.
Future Prospects and Challenges
While aluminum’s valence electron configuration offers numerous advantages, there are also challenges to consider:
- Recycling: Aluminum’s recyclability is a significant advantage, as it reduces environmental impact. However, ensuring proper recycling practices and infrastructure is crucial to maximize its benefits.
- Sustainability: As the demand for aluminum continues to grow, sustainable sourcing and production methods become increasingly important. Addressing these sustainability concerns is essential for the long-term viability of aluminum.
- Innovation: The unique properties of aluminum continue to drive innovation in various fields. Researchers and engineers are exploring new applications and improvements, pushing the boundaries of what aluminum can achieve.
Final Thoughts
Aluminum’s valence electrons, with their incomplete third shell, unlock a world of possibilities and challenges. From its high reactivity to its versatility and corrosion resistance, aluminum’s unique electron configuration has made it an indispensable element in modern society. Understanding the role of valence electrons not only provides insights into aluminum’s behavior but also highlights the fascinating intricacies of the atomic world.
Aluminum's valence electrons, with their incomplete third shell, drive its reactivity, versatility, and unique properties, making it a cornerstone element in various industries.
How many valence electrons does aluminum have?
+Aluminum has three valence electrons in its outermost energy level or valence shell.
What is the significance of aluminum’s valence electrons?
+Aluminum’s three valence electrons make it highly reactive, allowing it to form a wide range of compounds and play a crucial role in various industries.
How does aluminum’s reactivity affect its applications?
+Aluminum’s reactivity makes it versatile, suitable for construction, transportation, and packaging, but it also poses challenges in certain environments, such as corrosion concerns.
What are the benefits of aluminum’s electrical conductivity?
+Aluminum’s electrical conductivity makes it useful for electrical transmission lines and wiring, but it can also be a drawback in certain applications where insulation is required.
What are the future prospects for aluminum in terms of sustainability and innovation?
+The future of aluminum lies in sustainable sourcing, efficient recycling practices, and continued innovation, ensuring its long-term viability and impact in various industries.



