The Ultimate Guide to Trigonal Bipyramidal Geometry
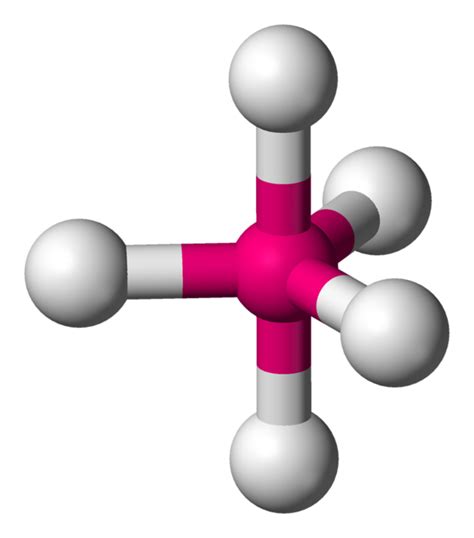
Trigonal bipyramidal geometry is a fascinating molecular arrangement that showcases the elegance and complexity of molecular architecture. This geometry, often abbreviated as TBP, is a five-vertex structure with a unique symmetry and a distinct visual appeal. Let’s dive into the intricacies of this geometry and explore its applications and significance in the world of chemistry.
The trigonal bipyramidal geometry is characterized by five atoms or groups of atoms arranged around a central atom. This arrangement forms a unique shape, resembling a pyramid with a triangular base and two additional atoms positioned above and below the base. The resulting structure has a pentagonal symmetry, creating a visually striking and highly symmetrical molecular configuration.
Historical Evolution of TBP Understanding

The concept of trigonal bipyramidal geometry has its roots in early studies of molecular structure and bonding. As chemists delved deeper into understanding the behavior of atoms and their interactions, the unique geometry of TBP emerged as a distinct possibility. Over time, with advancements in technology and theoretical frameworks, the TBP geometry gained recognition and became a well-established concept in the field of chemistry.
The historical evolution of TBP understanding can be traced back to the pioneering work of scientists like Linus Pauling and Gilbert N. Lewis, who laid the foundation for modern molecular theories. Their contributions, along with subsequent research, led to a better comprehension of the electronic configurations and bonding patterns that give rise to TBP geometry.
Theoretical Foundations: Understanding Electron Configuration

At the heart of trigonal bipyramidal geometry lies the concept of electron configuration and bonding. The TBP structure arises when a central atom, typically with a valence shell electron configuration of sp^3d, forms bonds with five other atoms or groups. This unique electron configuration allows for the formation of five covalent bonds, resulting in the distinctive TBP arrangement.
The electron configuration of the central atom plays a crucial role in determining the geometry. In the case of TBP, the sp^3d hybridization involves the mixing of one s, three p, and one d atomic orbitals to create five hybrid orbitals. These hybrid orbitals then align themselves to accommodate the five bonding pairs of electrons, leading to the characteristic TBP geometry.
Bonding Patterns and Interactions
The bonding pattern in TBP geometry is intricate and fascinating. The five atoms or groups surrounding the central atom can be arranged in two distinct ways, leading to different bonding interactions. These arrangements are known as the axial and equatorial positions.
In the axial positions, two atoms or groups are located above and below the triangular base, forming a linear arrangement with the central atom. These axial bonds are often characterized by stronger interactions and shorter bond lengths compared to the equatorial bonds.
The equatorial positions, on the other hand, are situated on the triangular base, forming an angle of 120^\circ with each other. These bonds are slightly weaker and longer compared to the axial bonds due to the increased steric hindrance. The interplay between axial and equatorial bonds adds complexity to the TBP geometry, influencing its reactivity and properties.
Symmetry and Molecular Properties
The trigonal bipyramidal geometry boasts a high degree of symmetry, which has significant implications for its molecular properties. The symmetry of TBP gives rise to unique vibrational and rotational patterns, affecting the molecule’s behavior in various physical and chemical processes.
The symmetry elements in TBP include a C_3 rotation axis and a S_2 inversion center. These symmetry elements dictate the molecule’s behavior under certain transformations, such as rotations and reflections. The presence of these symmetry elements influences the molecule’s spectral properties, making TBP a fascinating subject for spectroscopic studies.
Applications and Real-World Examples

Trigonal bipyramidal geometry finds applications in various fields, showcasing its versatility and importance. Here are some real-world examples where TBP geometry plays a crucial role:
Transition Metal Complexes: Many transition metal complexes adopt TBP geometry, particularly in their coordination compounds. The unique arrangement of ligands around the central metal ion results in interesting magnetic and electronic properties, making them valuable in catalysis and materials science.
Phosphorus Compounds: Phosphorus, with its sp^3d hybridization, often forms trigonal bipyramidal structures. Phosphorus pentachloride (PCl_5) and phosphorus pentoxide (P_2O_5) are classic examples of TBP geometry, showcasing the versatility of phosphorus in forming stable compounds.
Organometallic Chemistry: In organometallic compounds, TBP geometry is observed when a metal atom coordinates with five ligands. These compounds find applications in various fields, including catalysis, materials synthesis, and even medicine.
Natural Products: Some naturally occurring compounds exhibit TBP geometry. For instance, certain alkaloids and terpenes display TBP structures, contributing to their unique biological activities and interactions with other molecules.
Practical Considerations: Steric Hindrance and Bonding Strength
While TBP geometry is visually appealing and theoretically fascinating, practical considerations come into play when working with molecules of this geometry. Steric hindrance, or the spatial arrangement of atoms, can significantly impact the stability and reactivity of TBP compounds.
The axial positions, with their shorter bond lengths, often experience greater steric hindrance compared to the equatorial positions. This can lead to differences in reactivity, with axial bonds potentially being more susceptible to certain reactions or interactions. Understanding these practical considerations is crucial for chemists working with TBP compounds in synthetic and analytical chemistry.
Comparative Analysis: TBP vs. Other Geometries
Trigonal bipyramidal geometry is just one of many molecular geometries, each with its unique characteristics and applications. Here’s a comparative analysis of TBP with some other common geometries:
| Geometry | Number of Atoms | Bond Angles | Symmetry | Common Examples |
|---|---|---|---|---|
| Trigonal Bipyramidal | 5 | 90^\circ (axial), 120^\circ (equatorial) | Pentagonal | Phosphorus pentachloride, Sulfur hexafluoride |
| Tetrahedral | 4 | 109.5^\circ | Tetrahedral | Methane, Carbon tetrachloride |
| Octahedral | 6 | 90^\circ | Octahedral | Hexaaquacopper(II) ion, Sulfur hexafluoride |
| Linear | 2 | 180^\circ | Linear | Carbon dioxide, Hydrogen gas |
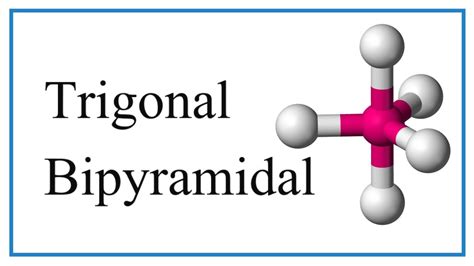
As seen in the table, each geometry has its distinct features and is suited for different molecular arrangements. The choice of geometry depends on the central atom’s electron configuration, the number of bonding pairs, and the desired properties of the resulting molecule.
Future Trends and Emerging Applications
The study of trigonal bipyramidal geometry continues to evolve, with new research and applications emerging. Here are some future trends and potential areas of exploration:
Green Chemistry: TBP geometry can play a role in developing sustainable and environmentally friendly chemical processes. Understanding the reactivity and selectivity of TBP compounds can lead to more efficient and eco-friendly reactions.
Medicinal Chemistry: The unique bonding patterns and properties of TBP compounds make them attractive targets for medicinal chemistry. Exploring TBP-based molecules as potential drug candidates could open new avenues for pharmaceutical research.
Materials Science: TBP geometry, particularly in transition metal complexes, offers opportunities for the development of advanced materials with unique electronic and magnetic properties. These materials could find applications in electronics, energy storage, and catalysis.
Conclusion
Trigonal bipyramidal geometry is a captivating and essential concept in the field of chemistry. Its unique symmetry, bonding patterns, and applications make it a subject of ongoing research and exploration. By understanding the theoretical foundations, practical considerations, and real-world implications, we can appreciate the beauty and significance of TBP geometry in molecular architecture.
As our knowledge of molecular structures expands, the study of trigonal bipyramidal geometry will continue to contribute to our understanding of the chemical world, offering insights and applications that benefit various scientific disciplines.
What is the significance of the axial and equatorial positions in TBP geometry?
+The axial and equatorial positions in TBP geometry play a crucial role in determining the molecule’s reactivity and properties. Axial bonds are often stronger and shorter, making them more reactive, while equatorial bonds experience increased steric hindrance.
Can all elements form trigonal bipyramidal structures?
+No, not all elements can form TBP structures. The ability to adopt TBP geometry depends on the element’s electron configuration and its capacity to hybridize and form five bonds. Elements like phosphorus, sulfur, and certain transition metals are more likely to exhibit TBP geometry.
What are some practical applications of TBP geometry in everyday life?
+TBP geometry finds applications in various industries. For example, TBP compounds are used in the production of fertilizers, pesticides, and pharmaceuticals. Additionally, TBP-based materials can be found in electronics and energy storage devices.
How does TBP geometry influence the magnetic properties of molecules?
+The TBP geometry, particularly in transition metal complexes, can affect the molecule’s magnetic properties. The arrangement of ligands and the resulting electron distribution can lead to interesting magnetic behaviors, making TBP compounds valuable in materials science and spintronics.
Are there any limitations or challenges associated with TBP geometry in synthetic chemistry?
+Yes, one challenge is the steric hindrance associated with TBP geometry. The axial positions, with their shorter bond lengths, can experience greater steric strain, making the synthesis of certain TBP compounds more complex. However, with careful design and understanding of the molecular interactions, these challenges can be overcome.