Titanium's Atomic Dance: Electron Configuration Unveiled

Titanium, a metal with a fascinating atomic structure, boasts an electron configuration that plays a pivotal role in its unique properties and wide-ranging applications. Delving into the intricacies of this configuration reveals a complex dance of electrons, each with its own distinct movements and interactions.
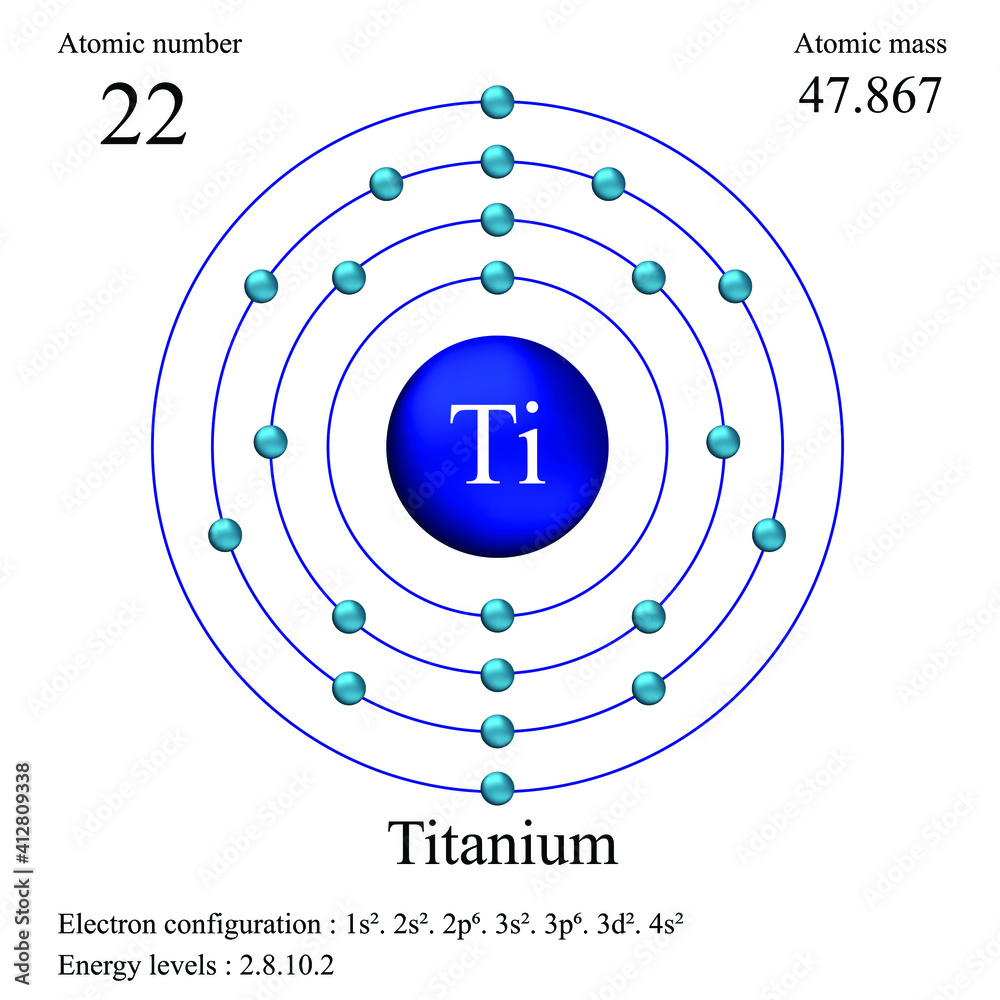
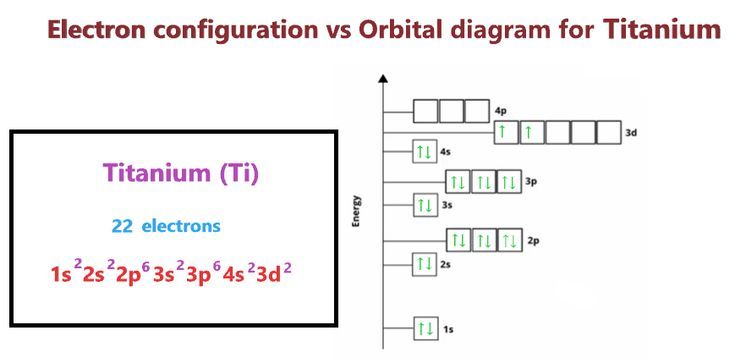
Titanium, with its atomic number 22, presents an electron configuration that tells a story of precision and complexity. This configuration, [Ar] 3d2 4s2, reflects the element’s placement in the periodic table and hints at the secrets of its behavior.
The Atomic Orchestra
Visualize titanium’s electron configuration as an intricate musical score, with each electron a unique instrument in an atomic orchestra. The noble gas argon, represented by [Ar], sets the foundation, providing a stable base for the higher-energy electrons to build upon.
The two electrons in the 3d orbital dance in a harmonious duo, their movements influenced by the surrounding atomic environment. Meanwhile, the 4s electrons contribute a contrasting rhythm, each following its own path yet contributing to the overall melody.
A Balanced Act
Titanium’s electron configuration showcases a delicate balance between stability and reactivity. The partially filled 3d orbital, with its two electrons, hints at a desire for completion, a drive that contributes to titanium’s propensity for forming strong bonds with other elements.
This balance is a testament to titanium’s versatility, a metal that can be both strong and flexible, stable yet reactive when required.
Historical Perspective
Understanding titanium’s electron configuration has been a gradual process, building upon the foundations laid by early pioneers in atomic theory. The development of quantum mechanics provided the theoretical framework, allowing scientists to decipher the complex interactions within the atom.
A Historical Timeline:
- 1913: Niels Bohr proposes the Bohr model, a breakthrough in atomic theory, laying the groundwork for understanding electron configurations.
- 1926: Erwin Schrödinger develops the Schrödinger equation, providing a mathematical description of electron behavior, a critical tool in deciphering configurations.
- 1930s: With the advent of quantum mechanics, scientists begin to unravel the complexities of electron configurations, including titanium's unique arrangement.
- 1950s-1960s: Experimental techniques, such as X-ray spectroscopy, provide empirical evidence supporting the theoretical models of electron configurations.
Modern Applications
Today, titanium’s electron configuration continues to be a driving force behind its widespread use. From aerospace engineering to medical implants, the element’s unique atomic structure lends it remarkable properties.
Why is titanium preferred for aerospace applications?
+Titanium's high strength-to-weight ratio, a direct result of its electron configuration, makes it ideal for aerospace components. Its resistance to corrosion and fatigue further enhances its suitability, ensuring structural integrity over extended periods.
How does titanium's electron configuration contribute to its biocompatibility?
+The stability provided by titanium's electron configuration, coupled with its ability to form strong bonds, results in a surface that is highly biocompatible. This makes titanium an excellent choice for medical implants, where long-term stability and minimal rejection are crucial.
Can titanium's electron configuration be manipulated to enhance its properties?
+Absolutely. By alloying titanium with other elements, we can influence its electron configuration, leading to tailored properties. For instance, adding vanadium can enhance its strength and toughness, making it even more versatile.
What are the challenges associated with titanium's electron configuration in certain applications?
+While titanium's electron configuration offers many advantages, it can also present challenges. Its limited conductivity, for instance, may restrict its use in certain electronic applications. Additionally, its reactivity with certain materials requires careful consideration during manufacturing and use.
Titanium’s atomic dance, as revealed through its electron configuration, showcases the intricate beauty of the atomic world. This understanding continues to drive innovation, pushing the boundaries of what this remarkable metal can achieve.



