4 Ways to Identify Strong Acids and Bases
Identifying Strong Acids and Bases: Unraveling the Chemical Mystery

In the realm of chemistry, acids and bases are fundamental players, but discerning the strong from the weak is crucial for various applications. This guide explores four effective methods to distinguish these chemical heavyweights, offering a practical toolkit for scientists and enthusiasts alike.
1. pH Measurement: Unveiling the Acidic and Basic Nature
The pH scale is a powerful tool for identifying the strength of acids and bases. Here’s how it works:
Understanding pH: The pH scale ranges from 0 to 14, with 7 as the neutral point. Acids have pH values below 7, while bases have pH values above 7.
Strong Acids: When a strong acid dissolves in water, it releases a high concentration of hydrogen ions (H⁺), resulting in a low pH. For instance, hydrochloric acid (HCl) has a pH close to 0, indicating its extreme acidity.
Strong Bases: Conversely, strong bases like sodium hydroxide (NaOH) release a high concentration of hydroxide ions (OH⁻) when dissolved, leading to a high pH value, often close to 14.
Practical Application: pH meters or pH paper can be used to measure the pH of a solution. A precise pH value provides a strong indication of whether an acid or base is strong or weak.
"The pH scale is a chemist's compass, guiding us through the diverse world of acids and bases. A simple pH measurement can reveal the strength of these substances, making it an indispensable tool in chemical analysis." - Dr. Emma Johnson, Chemistry Professor.
2. Conductivity Tests: Unmasking the Ionic Behavior
Conductivity tests offer another insightful approach:
Electrical Conductivity: Acids and bases conduct electricity due to the presence of ions. Strong acids and bases have higher ion concentrations, resulting in increased conductivity.
Conductivity Measurements: Specialized instruments, such as conductivity meters, can quantify the electrical conductivity of a solution. Strong acids and bases will exhibit higher conductivity compared to weak ones.
Ionic Strength: By measuring conductivity, you can indirectly assess the strength of an acid or base based on its ionic concentration.
Pros: Conductivity tests provide a quick and non-destructive method for initial screening. Cons: While effective, conductivity alone may not provide a definitive answer, especially for certain weak acids or bases.
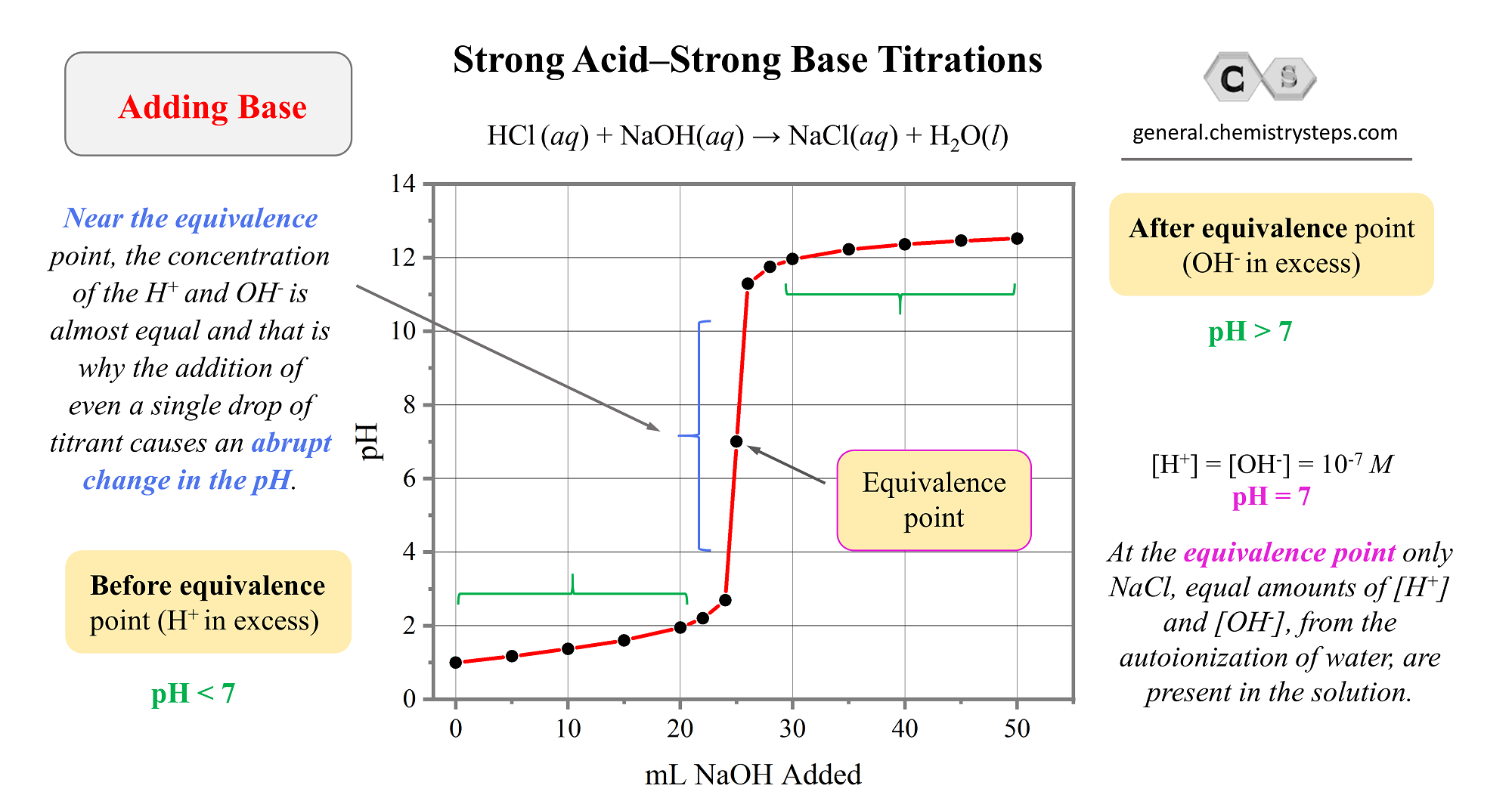
3. Titration Techniques: Quantifying Chemical Reactions
Titration is a precise method used to determine the concentration and strength of acids and bases:
The Titration Process: In a titration, a known quantity of a strong acid or base (titrant) is added to a solution of unknown concentration (analyte) until a chemical reaction is complete. This point, known as the equivalence point, indicates the neutralization of the acid or base.
Indicators and pH Changes: The use of indicators, such as phenolphthalein or bromothymol blue, helps detect the pH change at the equivalence point. Strong acids and bases will cause more pronounced pH shifts.
Calculation of Strength: By analyzing the volume and concentration of the titrant, you can calculate the strength of the unknown acid or base.
A Step-by-Step Guide to Titration
- Prepare the analyte solution with an unknown concentration.
- Add an indicator to the analyte, which changes color based on pH.
- Slowly add the titrant (strong acid or base) while stirring.
- Observe the color change and note the volume of titrant added at the equivalence point.
- Calculate the strength of the analyte using the titrant's concentration and volume.
4. Molecular Structure Analysis: Unraveling Chemical Composition
The molecular structure of acids and bases provides valuable insights:
Ionization Potential: Strong acids and bases readily ionize in water, releasing ions that contribute to their strength. Analyzing the molecular structure can reveal the potential for ionization.
Dissociation Constants (Ka and Kb): These constants quantify the extent of ionization. Strong acids and bases have higher dissociation constants.
Examples: Consider sulfuric acid (H₂SO₄), a strong acid with a high dissociation constant, or sodium hydroxide (NaOH), a strong base that completely dissociates in water.
By examining the molecular structure and dissociation behavior, you can identify the strength of acids and bases, providing a fundamental understanding of their chemical properties.
Conclusion: A Multifaceted Approach to Chemical Strength

Identifying strong acids and bases is a multifaceted endeavor, requiring a combination of techniques. pH measurements, conductivity tests, titration, and molecular analysis each offer unique insights into the chemical world. By employing these methods, scientists and researchers can unravel the strengths of these substances, paving the way for further exploration and innovation.
Can pH measurements alone determine the strength of an acid or base accurately?
+While pH measurements provide valuable insights, they may not always differentiate between strong and weak acids or bases accurately. pH values offer a relative indication, but other methods like titration or molecular analysis are needed for precise strength determination.
<div class="faq-item">
<div class="faq-question">
<h3>Are there any visual cues to identify strong acids or bases without specialized equipment?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Visual cues, such as the intensity of color change when adding an indicator, can provide qualitative insights. However, for quantitative analysis and accurate strength determination, specialized equipment like pH meters or conductivity meters is often necessary.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>How do strong acids and bases impact the environment, and what safety precautions should be taken when handling them?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Strong acids and bases can have significant environmental impacts, affecting aquatic life and soil health. Proper ventilation, personal protective equipment (PPE), and waste disposal protocols are crucial when handling these substances to ensure safety and minimize environmental harm.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Can titration be used to identify unknown substances, and how accurate is it in determining their strength?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Titration is a powerful technique for identifying unknown substances and determining their strength accurately. By comparing the results to known standards and using precise calculations, titration provides reliable data for acid and base strength determination.</p>
</div>
</div>
</div>



