The Top 5 Standard Reduction Potential Table Insights
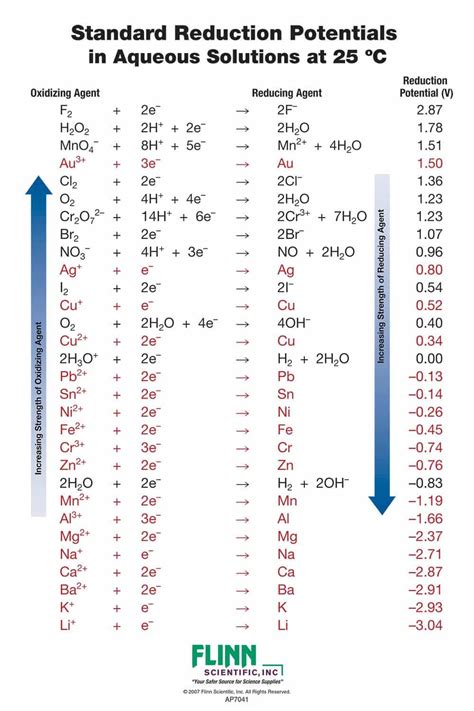
Understanding the Language of Electrochemistry

The Standard Reduction Potential Table, often referred to as the “Redox Table,” is a cornerstone of electrochemistry, providing a wealth of insights into the behavior of elements and compounds in redox reactions. This table serves as a guide, offering a systematic way to predict and understand the flow of electrons in chemical reactions. Let’s delve into the top five insights this table provides, exploring the intricacies of electron transfer and its impact on various chemical processes.
1. Unveiling the Hierarchy of Electron Transfer
The primary function of the Standard Reduction Potential Table is to rank elements and compounds based on their propensity to gain or lose electrons. This ranking establishes a clear hierarchy, revealing which substances are the strongest oxidizing agents (those that readily lose electrons) and which are the most potent reducing agents (those that readily accept electrons). By organizing elements and compounds in this manner, the table provides a fundamental understanding of the electron transfer process, a key aspect of redox reactions.
The beauty of the Redox Table lies in its ability to simplify complex electron transfer processes, offering a visual representation of the electron donation and acceptance hierarchy.
2. Predicting Reaction Spontaneity
A crucial insight from the Standard Reduction Potential Table is its ability to predict the spontaneity of redox reactions. By comparing the reduction potentials of reactants and products, chemists can determine whether a reaction is likely to occur spontaneously. If the reduction potential of the reactants is more negative than that of the products, the reaction is thermodynamically favorable and will proceed spontaneously. This predictive power is invaluable in designing and optimizing chemical processes.
Pros of Using the Redox Table for Spontaneity Prediction
- Provides a quick and reliable method to assess reaction feasibility.
- Aids in the design of energy-efficient chemical processes.
Cons to Consider
- Does not account for kinetic factors, which can influence reaction rates.
- Relies on standard conditions, which may differ from real-world scenarios.
3. Unlocking Electrochemical Cell Potential
The Redox Table is a key tool in understanding and designing electrochemical cells. These cells, whether galvanic or electrolytic, rely on the difference in reduction potentials between the cathode and anode to generate electrical potential. By selecting the appropriate half-reactions from the table, chemists can construct cells with specific voltage outputs, making the table essential for applications ranging from batteries to electroplating.
The Standard Reduction Potential Table is a powerful resource for electrochemical engineering, enabling the design of efficient and targeted electrochemical systems.
4. Insights into Element Behavior
Beyond its practical applications, the Redox Table offers profound insights into the inherent behavior of elements. It reveals the electron affinity and ionization energy trends across the periodic table, providing a deeper understanding of elemental properties. This knowledge is instrumental in various fields, from materials science to environmental chemistry, where the behavior of elements under different conditions is crucial.
| Element Group | Electron Affinity | Ionization Energy |
|---|---|---|
| Alkali Metals | Low (Readily lose electrons) | Low (Easily ionize) |
| Noble Gases | High (Resist gaining electrons) | High (Resist ionization) |
| Transition Metals | Variable (Dependent on oxidation state) | Moderate to High (Some are harder to ionize) |

5. A Foundation for Environmental Chemistry
In the realm of environmental chemistry, the Standard Reduction Potential Table plays a critical role in understanding and addressing environmental issues. It provides insights into the redox behavior of pollutants and contaminants, guiding the development of remediation strategies. For instance, understanding the reduction potential of heavy metals aids in their removal from water bodies, a crucial step in environmental conservation.
Applying the Redox Table in Environmental Remediation
- Identify the pollutant and its reduction potential.
- Select an appropriate reducing agent with a lower reduction potential to drive the reaction.
- Implement the chosen reducing agent to convert the pollutant into a less harmful form.
- Monitor and adjust as needed to ensure effective remediation.
FAQ Section
How does the Standard Reduction Potential Table aid in battery design?
+The Redox Table is crucial for battery design as it allows engineers to select electrode materials with specific reduction potentials. This ensures the battery has the desired voltage output and energy storage capacity, making it an essential tool for developing efficient and reliable energy storage solutions.
Can the table predict reaction kinetics, or just spontaneity?
+While the Standard Reduction Potential Table provides insights into reaction spontaneity, it does not directly predict reaction kinetics. Reaction rates are influenced by various factors, including temperature, concentration, and activation energy barriers. However, the table serves as a starting point for understanding the thermodynamic feasibility of reactions.
What are the implications of an element's position in the Redox Table for its environmental impact?
+The position of an element in the Redox Table can indicate its potential environmental impact. Elements with high reduction potentials, indicating a strong tendency to lose electrons, may pose environmental risks due to their reactivity. Proper handling and disposal are crucial to mitigate these risks, especially in industrial and manufacturing processes.
How does the table help in the development of corrosion-resistant materials?
+The Redox Table is instrumental in the development of corrosion-resistant materials. By understanding the reduction potentials of various elements and compounds, material scientists can design alloys and coatings that have reduction potentials favorable for corrosion resistance. This ensures the longevity and durability of materials in corrosive environments.
In conclusion, the Standard Reduction Potential Table is a multifaceted tool, offering a deep understanding of electron transfer, reaction spontaneity, and the inherent behavior of elements. Its applications span from the design of efficient electrochemical cells to the development of environmental remediation strategies, making it an indispensable resource in the field of chemistry.



