Mastering the SF4 Lewis Structure in 5 Steps

Unraveling the Complexity of the SF4 Lewis Structure
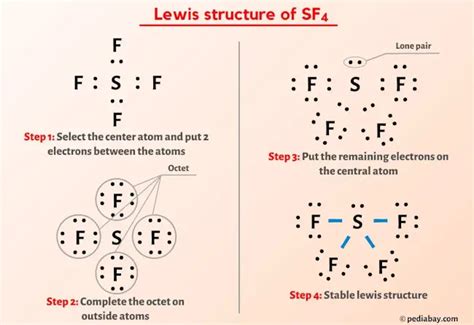
The SF4 Lewis Structure, often an enigmatic concept for many chemistry enthusiasts, is actually quite manageable once you grasp the underlying principles. In this comprehensive guide, we’ll demystify the process, making it accessible and intuitive. Let’s embark on this journey towards mastery!
Step 1: Understanding the Basics
At its core, the Lewis Structure, named after its inventor, Gilbert N. Lewis, provides a pictorial representation of the distribution of electrons within a molecule. For SF4, a molecule with a unique tetrahedral shape, this structure becomes particularly intriguing.
Start by recognizing that sulfur, being the central atom, is from the third period of the periodic table, which means it can accommodate more electrons than the typical two in its outermost shell.
The periodic table offers a valuable roadmap, guiding us to anticipate the electron behavior of different elements.
Each fluorine atom, on the other hand, from the second period, seeks a stable configuration akin to the noble gas Neon, necessitating a maximum of eight electrons in its outermost shell.
Step 2: Counting Valence Electrons
The next step involves a simple yet crucial calculation: counting the valence electrons. For sulfur, with six valence electrons, and fluorine, with seven each, the math is straightforward. In total, SF4 boasts 34 valence electrons.
This number becomes a foundational element in our subsequent steps, influencing the very structure of the molecule.
Step 3: Constructing the Framework
With the valence electrons counted, it's time to build the molecular framework. Place the sulfur atom at the center, and around it, arrange the four fluorine atoms, forming a tetrahedral structure. This arrangement reflects the molecule's unique shape.
As we connect the dots, literally, we begin to visualize the electron distribution, a critical step towards understanding the molecule's behavior.
Step 4: Bonding and Lone Pairs
Now, the fun part begins: pairing up electrons. Each bond between sulfur and fluorine utilizes two electrons, reducing our count of available electrons. With eight bonds formed, we're left with a substantial number of lone pairs.
These lone pairs, residing around the fluorine atoms, play a pivotal role in determining the molecule's properties and reactivity.
A key takeaway: understanding the distribution of these lone pairs is essential for predicting the molecule's behavior in various chemical reactions.
Step 5: Finalizing the Structure
In the final step, we refine our structure. Check that each atom's octet is satisfied, ensuring stability. If any deviations occur, adjust the structure accordingly.
At this stage, the Lewis Structure of SF4 takes shape, offering a comprehensive view of the molecule's electron configuration.
By following these five steps, you've not only mastered the SF4 Lewis Structure but also gained a deeper insight into the fundamental principles of chemistry.
Exploring Further: The Practical Implications
Mastery of the SF4 Lewis Structure opens doors to a world of practical applications. Understanding electron distribution becomes a powerful tool for predicting chemical reactions, designing new compounds, and optimizing industrial processes.
Imagine a chemist, armed with this knowledge, devising strategies to enhance the efficiency of sulfur-based fertilizers or developing innovative fluorinated compounds for medical applications. The possibilities are vast and intriguing.
Conclusion: A Journey of Discovery
The SF4 Lewis Structure, once an enigma, has revealed its secrets. Through this journey, we’ve uncovered the elegance of molecular structures and the profound insights they offer.
As we conclude, remember that the world of chemistry is vast and ever-evolving. Each new concept, like the SF4 Lewis Structure, adds a piece to the grand puzzle, inviting us to explore further and discover more.
What makes the SF4 Lewis Structure unique compared to other molecules?
+The SF4 Lewis Structure stands out due to the central sulfur atom’s ability to accommodate more electrons, resulting in a unique tetrahedral shape. This structure contrasts with the more common linear or trigonal planar configurations seen in many other molecules.
How does understanding the SF4 Lewis Structure benefit real-world applications?
+Knowledge of the SF4 Lewis Structure allows chemists to predict and manipulate the molecule’s behavior. This insight is invaluable for developing new materials, optimizing chemical processes, and even designing more effective pharmaceuticals.
Can the Lewis Structure be used for all chemical compounds?
+Absolutely! The Lewis Structure is a versatile tool applicable to a wide range of chemical compounds. While the specific steps may vary depending on the molecule’s complexity, the fundamental principles remain consistent.
What challenges might one encounter when constructing the SF4 Lewis Structure?
+A common challenge is ensuring each atom’s octet is satisfied. This involves a careful balance of bonding electrons and lone pairs. Additionally, the unique shape of SF4 may require some adjustments to achieve a stable structure.
Are there any online tools or software that can assist in constructing Lewis Structures?
+Indeed, several online platforms and software applications offer tools for constructing Lewis Structures. These resources can be particularly helpful for beginners or when dealing with complex molecules. However, manual construction is essential for developing a deep understanding.