The Ultimate Guide: Polyatomic Ions
Understanding Polyatomic Ions: A Comprehensive Exploration
Polyatomic ions, often referred to as molecular ions, are fascinating entities in the realm of chemistry. These ions consist of multiple atoms bonded together, forming unique structures with distinct properties. In this in-depth guide, we will embark on a journey to uncover the intricacies of polyatomic ions, exploring their formation, characteristics, and significant roles in various chemical processes.
The Building Blocks: Understanding Atomic and Ionic Bonds
To grasp the concept of polyatomic ions, we must first delve into the fundamental building blocks of matter: atoms and ions. Atoms, the smallest units of an element, are composed of protons, neutrons, and electrons. When atoms interact, they can form chemical bonds, resulting in molecules or ions.
Ionic bonds occur when one atom transfers one or more electrons to another atom, creating positively charged ions (cations) and negatively charged ions (anions). This transfer of electrons is driven by the desire to achieve a stable electron configuration, often resembling the nearest noble gas. For example, sodium (Na) loses an electron to form Na+, while chlorine (Cl) gains an electron to form Cl-.
Formation of Polyatomic Ions: A Molecular Journey
Polyatomic ions take this concept a step further by involving multiple atoms bonded together through covalent and/or ionic bonds. These complex ions exhibit unique behaviors and properties due to their molecular nature. Let’s explore some common mechanisms of polyatomic ion formation:
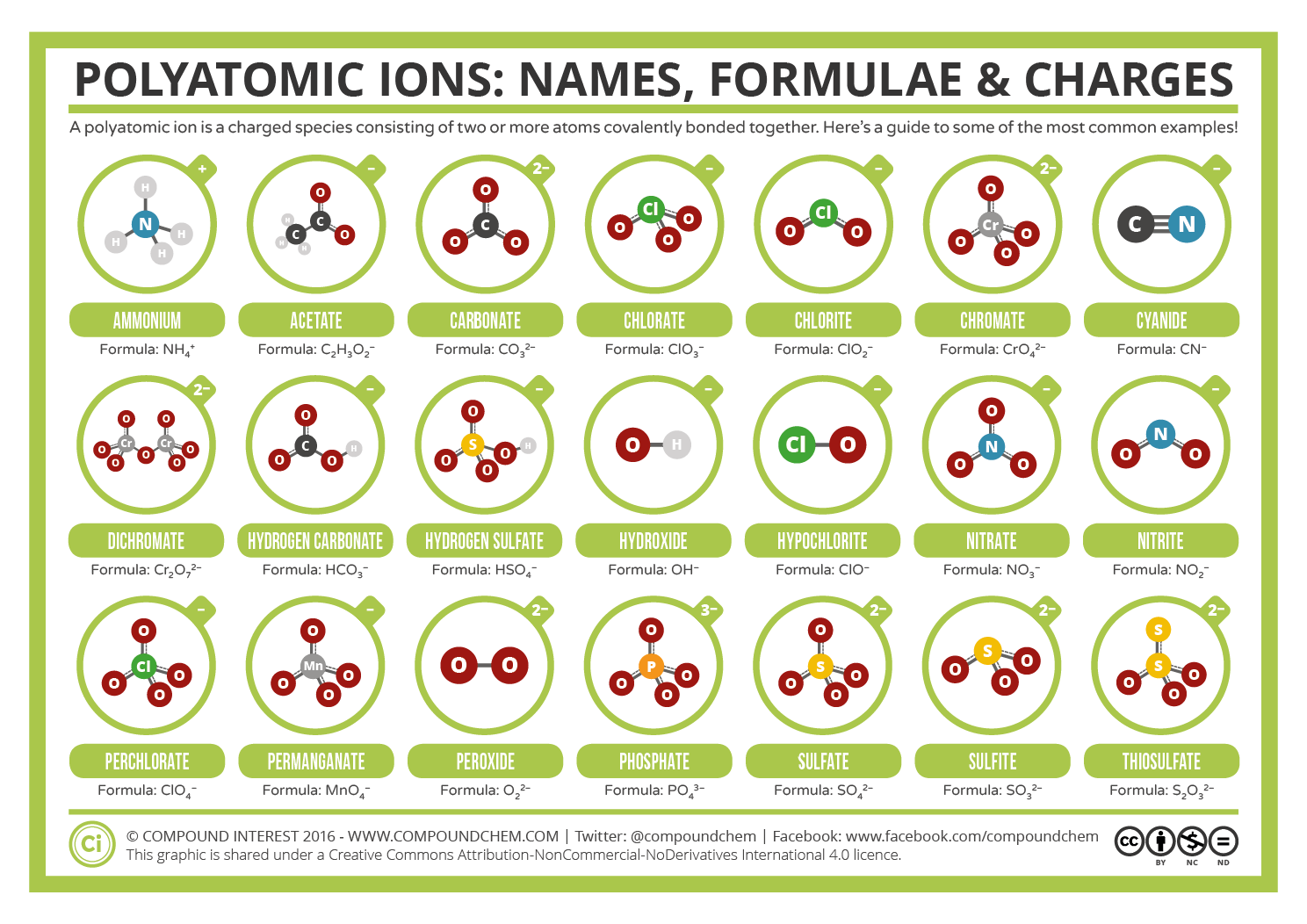
Covalent Bonding: In some cases, polyatomic ions are formed solely through covalent bonding, where atoms share electrons to achieve stability. For instance, the oxygen molecule (O2) is a diatomic molecule with a double covalent bond, and it can further combine with other atoms to form polyatomic ions like sulfate (SO4^2-) or nitrate (NO3^-).
Ionic Bonding with Molecules: Polyatomic ions can also be formed when a molecule gains or loses electrons to attain a stable charge. Take the case of the ammonium ion (NH4+), which is formed when the nitrogen atom in ammonia (NH3) accepts a proton (H+), resulting in a positively charged ion.
Combination of Ionic and Covalent Bonds: Many polyatomic ions exhibit a blend of ionic and covalent bonding. For example, the carbonate ion (CO3^2-) consists of a central carbon atom bonded to three oxygen atoms through covalent bonds, and the entire ion carries a negative charge due to the ionic bond with additional electrons.
Structural Diversity and Naming Conventions
Polyatomic ions showcase an incredible range of structural diversity, with varying shapes, sizes, and compositions. This complexity gives rise to the need for systematic naming conventions to identify and categorize these ions accurately. Here’s a glimpse into the world of polyatomic ion nomenclature:
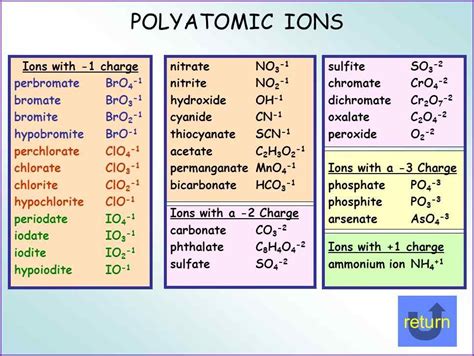
Root Words and Affixes: The names of polyatomic ions often reflect their composition and charge. For instance, the root word “sulfate” indicates the presence of sulfur and oxygen, while the suffix “-ate” signifies a negative ion. Similarly, “nitrite” suggests the presence of nitrogen and oxygen, with the suffix “-ite” indicating a less electronegative element.
Common Names and IUPAC Nomenclature: Some polyatomic ions have common names that are widely recognized, such as “phosphate” for PO4^3-. However, for more complex ions or to ensure consistency, the International Union of Pure and Applied Chemistry (IUPAC) has developed a comprehensive system for naming polyatomic ions. This system utilizes prefixes, suffixes, and systematic rules to construct unique and informative names.
Properties and Applications of Polyatomic Ions
Polyatomic ions exhibit a myriad of properties that make them indispensable in various chemical processes and applications. Here’s an overview of some key characteristics and their implications:
Chemical Reactivity: Polyatomic ions can participate in a wide range of chemical reactions, acting as reactants, products, or intermediates. Their molecular nature often allows for versatile bonding and interaction patterns, contributing to the richness of chemical reactions.
Solubility and Precipitation: The solubility of polyatomic ions in different solvents is a critical factor in various chemical processes. Understanding the solubility rules for polyatomic ions helps predict the formation of precipitates or the solubility of compounds. This knowledge is essential in fields like environmental chemistry, where ion exchange processes play a vital role.
Acids and Bases: Many polyatomic ions are integral components of acids and bases. For instance, the phosphate ion (PO4^3-) is a key player in phosphoric acid (H3PO4), a strong acid with numerous applications in industry and biology. Additionally, polyatomic ions like hydroxide (OH-) and carbonate (CO3^2-) are fundamental to the behavior of bases and their reactions.
Biological Significance: Polyatomic ions are ubiquitous in biological systems, playing crucial roles in enzyme activity, cellular signaling, and the overall functioning of living organisms. For example, phosphate ions are essential for energy storage and transfer in biological molecules like ATP.
Case Study: The Role of Polyatomic Ions in Environmental Chemistry
To illustrate the practical applications of polyatomic ions, let’s explore a case study focused on environmental chemistry.
Environmental scientists and engineers often encounter polyatomic ions when studying water quality and pollution control. One critical aspect is the analysis of nitrate (NO3^-) and nitrite (NO2^-) ions in water bodies. These ions, derived from various sources such as agricultural runoff or industrial waste, can have detrimental effects on aquatic ecosystems and human health.
By understanding the behavior and reactivity of nitrate and nitrite ions, researchers can develop strategies to mitigate their impact. For instance, certain polyatomic ions like sulfate (SO4^2-) can interact with nitrate ions to form less soluble compounds, facilitating their removal from water through precipitation.
Future Trends and Emerging Technologies
As our understanding of polyatomic ions deepens, so does our ability to harness their potential in emerging technologies. Here are some exciting avenues for future exploration:
Advanced Materials: Polyatomic ions, particularly those with unique structural properties, are being investigated for their potential in developing advanced materials. For example, research is underway to utilize polyatomic ions as building blocks for novel polymers with enhanced mechanical, electrical, or optical properties.
Energy Storage and Conversion: The study of polyatomic ions is pivotal in the development of efficient energy storage systems. For instance, polyatomic ions like lithium ions (Li+) play a central role in lithium-ion batteries, powering everything from portable electronics to electric vehicles. Understanding the behavior of these ions under different conditions is crucial for optimizing battery performance and safety.
Environmental Remediation: The environmental implications of polyatomic ions continue to drive research and innovation. Scientists are exploring innovative methods to remove or transform harmful polyatomic ions from contaminated environments. For example, the use of nanoparticles or advanced filtration techniques can selectively target and remove specific polyatomic ions, offering sustainable solutions for water purification and soil remediation.
Conclusion: Embracing the Complexity of Polyatomic Ions
In this comprehensive guide, we have embarked on a journey through the fascinating world of polyatomic ions. From their formation through covalent and ionic bonds to their diverse structures and properties, polyatomic ions offer a wealth of knowledge and opportunities.
As we conclude, it’s evident that polyatomic ions are not merely chemical entities but rather dynamic players in the intricate dance of matter. Their roles in chemistry, biology, and environmental science are profound, shaping the very fabric of our understanding and technological advancements.
By embracing the complexity and beauty of polyatomic ions, we open doors to endless possibilities, where science and innovation converge to create a brighter and more sustainable future.