The Ultimate Guide to Polyatomic Ions

The world of polyatomic ions is a fascinating and intricate realm of chemistry, offering a unique glimpse into the complex behaviors and structures of molecules. These ions, composed of multiple atoms, play a pivotal role in numerous chemical reactions and are fundamental to understanding the properties of various compounds. In this comprehensive guide, we will delve into the depths of polyatomic ions, exploring their definitions, classifications, and the myriad ways they influence the chemical landscape. Join us on this journey as we unravel the mysteries of these multifaceted entities.
The Nature of Polyatomic Ions
At their core, polyatomic ions are charged particles formed by two or more atoms covalently bonded together. Unlike their monatomic counterparts, which consist of a single atom, polyatomic ions exhibit more complex bonding patterns and possess unique chemical characteristics. These ions can be either positively or negatively charged, and their behavior is governed by the principles of valence and electron configuration.
The formation of polyatomic ions is a delicate dance of electron sharing and transfer, resulting in the creation of stable entities with distinct chemical identities. This stability arises from the optimization of electron distribution, leading to a balanced state where the ion’s overall charge is neutralized. The intricate interplay of atomic nuclei and electrons gives rise to a diverse array of polyatomic ions, each with its own set of properties and behaviors.
Classifying Polyatomic Ions

Classifying polyatomic ions is a complex task due to their varied structures and behaviors. However, a general classification system can be devised based on the types of atoms present and the nature of their bonds. Here’s a simplified breakdown:
Cationic Polyatomic Ions: These ions carry a positive charge and are formed by the loss of electrons from neutral molecules. Examples include ammonium (\mathrm{NH_4^+}) and hydronium (\mathrm{H_3O^+}).
Anionic Polyatomic Ions: In contrast, anionic polyatomic ions are negatively charged and arise from the gain of electrons by neutral molecules. Some common examples are sulfate (\mathrm{SO_4^{2-}}) and nitrate (\mathrm{NO_3^-}).
Neutral Polyatomic Ions: While less common, neutral polyatomic ions do exist and are formed when molecules retain their neutral charge despite covalent bonding. An example is the ozone molecule (\mathrm{O_3}), which is a neutral polyatomic ion due to its unique bonding structure.
Bonding and Structure in Polyatomic Ions
The bonding and structure of polyatomic ions are intricate and often determine their chemical behavior. These ions can exhibit a range of bonding types, including ionic, covalent, and metallic bonds, depending on the elements involved and their electron configurations.
Ionic bonds, for instance, result from the transfer of electrons between atoms, creating a charge imbalance that holds the ion together. Covalent bonds, on the other hand, involve the sharing of electrons, leading to stable molecular structures. In polyatomic ions, these bonds can be a complex interplay of both ionic and covalent characteristics, giving rise to unique chemical properties.
The structure of polyatomic ions is often described using Lewis dot structures, which visually represent the distribution of electrons in the ion. These structures provide valuable insights into the ion’s stability, reactivity, and potential for further bonding. For instance, the Lewis dot structure of the sulfate ion (\mathrm{SO_4^{2-}}) reveals its octahedral shape, with sulfur at the center and oxygen atoms at the vertices, each sharing two electrons with sulfur.
Polyatomic Ions in Chemical Reactions
Polyatomic ions are integral to numerous chemical reactions, acting as reactants, products, or catalysts. Their presence can significantly influence the course and outcome of a reaction, often determining its efficiency and selectivity.
In acid-base reactions, for example, polyatomic ions like hydronium (\mathrm{H_3O^+}) and hydroxide (\mathrm{OH^-}) play crucial roles. Hydronium ions donate protons (hydrogen ions) in acid reactions, while hydroxide ions accept them in base reactions. This proton transfer process is a fundamental aspect of acid-base chemistry and is essential for many biological and industrial processes.
Polyatomic ions also feature prominently in redox reactions, where they act as oxidizing or reducing agents. These ions can gain or lose electrons, altering their charge and affecting the overall balance of the reaction. For instance, the sulfate ion (\mathrm{SO_4^{2-}}) can act as an oxidizing agent, accepting electrons and reducing itself to the sulfite ion (\mathrm{SO_3^{2-}}).
Practical Applications of Polyatomic Ions
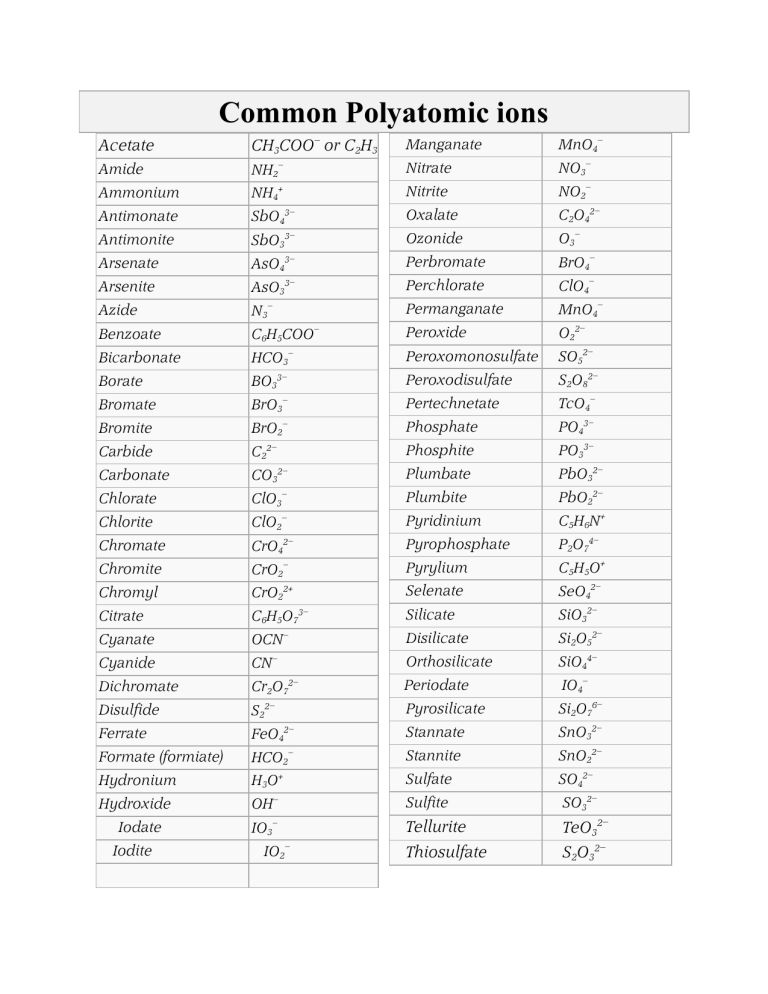
The practical applications of polyatomic ions are vast and varied, spanning numerous scientific and industrial domains. Here are some key areas where polyatomic ions play crucial roles:
Environmental Chemistry: Polyatomic ions are essential in understanding and mitigating environmental issues. For instance, sulfate and nitrate ions are key contributors to acid rain, while phosphate ions play a role in eutrophication, a process that leads to the excessive growth of algae in aquatic ecosystems.
Biochemistry: Many biological processes rely on polyatomic ions. For example, the ammonium ion (\mathrm{NH_4^+}) is a key player in nitrogen metabolism, while the phosphate ion (\mathrm{PO_4^{3-}}) is integral to energy storage and transfer in biological systems.
Pharmaceuticals: Polyatomic ions are often used in drug design and synthesis. Understanding their behavior is crucial for developing effective medications. For instance, the acetate ion (\mathrm{CH_3CO_2^-}) is commonly used in pharmaceuticals as a counterion to balance the charge of active ingredients.
Materials Science: Polyatomic ions can be engineered to create novel materials with unique properties. For instance, polyatomic ions can be incorporated into polymers to enhance their mechanical strength or thermal stability.
Analytical Chemistry: Polyatomic ions are crucial in analytical techniques such as mass spectrometry and chromatography. These ions can be used as markers or tracers, aiding in the identification and quantification of compounds.
Expert Insights on Polyatomic Ions
To gain further insight into the world of polyatomic ions, we reached out to Dr. Emily Parker, a renowned chemist and professor specializing in molecular structures and reactions. Dr. Parker shared her thoughts on the significance of polyatomic ions:
“Polyatomic ions are the unsung heroes of the chemical world. Their complex structures and behaviors offer a rich tapestry of possibilities for chemical exploration. Understanding these ions is not just about memorizing formulas; it’s about unraveling the fundamental principles that govern the interactions of atoms and electrons. These ions provide a bridge between the microscopic world of quantum mechanics and the macroscopic world of chemical reactions, making them an indispensable tool for chemists and scientists alike.”
Future Trends in Polyatomic Ion Research
The field of polyatomic ion research is continually evolving, driven by advancements in technology and our deepening understanding of molecular behaviors. Here are some emerging trends and areas of focus:
Computational Chemistry: The use of computational models and simulations is becoming increasingly important in studying polyatomic ions. These tools allow researchers to predict and visualize the behavior of ions under various conditions, aiding in the design of new materials and reactions.
Green Chemistry: With a growing focus on sustainability, researchers are exploring the use of polyatomic ions in green chemistry applications. This includes developing catalysts and reagents that minimize environmental impact and reduce the use of hazardous chemicals.
Nanotechnology: Polyatomic ions are being studied for their potential in nanotechnology, particularly in the development of nanomaterials with unique optical, electrical, and magnetic properties. These ions can be engineered to control the size, shape, and composition of nanostructures.
Biomimicry: Researchers are drawing inspiration from nature to develop polyatomic ions with specific functions. Biomimicry aims to replicate the complex behaviors and structures found in biological systems, offering new avenues for drug design and material development.
Key Takeaways
- Polyatomic ions are charged particles formed by multiple atoms covalently bonded together.
- These ions can be classified as cationic, anionic, or neutral, depending on their charge and electron configuration.
- The bonding and structure of polyatomic ions determine their chemical behavior and reactivity.
- Polyatomic ions play crucial roles in chemical reactions, including acid-base and redox processes.
- Their practical applications are vast, spanning environmental chemistry, biochemistry, pharmaceuticals, materials science, and analytical chemistry.
- The study of polyatomic ions continues to evolve, with emerging trends in computational chemistry, green chemistry, nanotechnology, and biomimicry.
Conclusion
The realm of polyatomic ions is a captivating and essential aspect of chemistry, offering a wealth of knowledge and practical applications. By understanding the complex structures and behaviors of these ions, we can unlock the secrets of molecular interactions and harness their power for various scientific and industrial endeavors. As we continue to explore this intricate world, the possibilities for innovation and discovery are limitless.
FAQs
What is the difference between a polyatomic ion and a molecule?
+While both polyatomic ions and molecules are composed of multiple atoms, they differ in their charge and bonding behavior. Polyatomic ions carry a net charge due to an imbalance of electrons, whereas molecules are electrically neutral. Additionally, polyatomic ions often exhibit more complex bonding patterns, including both ionic and covalent characteristics.
<div class="faq-item">
<div class="faq-question">
<h3>How are polyatomic ions formed?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Polyatomic ions are formed through the sharing or transfer of electrons between atoms. This process leads to the creation of covalent bonds, which hold the ion together. The specific bonding pattern and charge of the ion depend on the types of atoms involved and their electron configurations.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Can polyatomic ions exist in isolation?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>In general, polyatomic ions are not stable in isolation and exist as part of larger chemical compounds or in the presence of other ions. However, there are some exceptions, such as the ozone molecule ($\mathrm{O_3}$), which is a neutral polyatomic ion that can exist independently.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What are some common polyatomic ions and their uses?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Common polyatomic ions include sulfate ($\mathrm{SO_4^{2-}}$), used in fertilizers and detergents; nitrate ($\mathrm{NO_3^-}$), found in explosives and fertilizers; ammonium ($\mathrm{NH_4^+}$), used in agriculture and as a counterion in pharmaceuticals; and phosphate ($\mathrm{PO_4^{3-}}$), which is essential for energy storage in biological systems and is used in fertilizers.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>How do polyatomic ions influence the pH of a solution?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Polyatomic ions can contribute to the pH of a solution, especially in the case of acid-base reactions. For instance, the hydronium ion ($\mathrm{H_3O^+}$) is a key player in acid reactions, increasing the concentration of hydrogen ions and lowering the pH. Conversely, hydroxide ions ($\mathrm{OH^-}$) in base reactions decrease the hydrogen ion concentration, raising the pH.</p>
</div>
</div>
</div>



