The Ultimate Guide to Phosphate Lewis Structure

Phosphate is a fascinating and essential compound, playing a vital role in various biological and chemical processes. Understanding its Lewis structure provides valuable insights into its behavior and properties. In this comprehensive guide, we delve into the intricacies of the phosphate Lewis structure, exploring its fundamental concepts, step-by-step processes, and practical applications. Get ready to unravel the mysteries of this crucial molecule!
Unraveling the Phosphate Lewis Structure
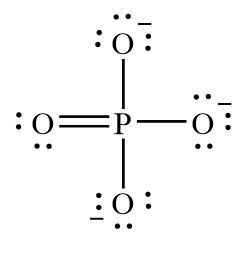
The Lewis structure, a fundamental concept in chemistry, allows us to visualize the arrangement of electrons in a molecule. In the case of phosphate, a negatively charged polyatomic ion, its Lewis structure provides a clear representation of its electronic configuration. Let’s break it down step by step:
Step 1: Identifying the Central Atom
Phosphate, with the chemical formula PO₄³⁻, consists of one phosphorus (P) atom surrounded by four oxygen (O) atoms. The phosphorus atom acts as the central atom due to its higher electronegativity and the need to satisfy its octet.
Step 2: Calculating Valence Electrons
To construct the Lewis structure, we must determine the total number of valence electrons available. Phosphorus, in group 15 of the periodic table, contributes five valence electrons, while each oxygen atom contributes six valence electrons. Thus, the total valence electrons for the phosphate ion are:
5 (P) + 4 x 6 (O) = 29 valence electrons
Step 3: Drawing the Skeleton Structure
We begin by placing the central phosphorus atom at the center of our diagram. Next, we distribute the oxygen atoms around the phosphorus, ensuring that each oxygen forms a single bond with the phosphorus atom. This gives us a preliminary skeleton structure:
- P
- ---O
- |
- |
- O O
Step 4: Satisfying Octets
Now, we focus on completing the octets of each atom. Phosphorus, with five valence electrons, requires three more electrons to achieve an octet. Similarly, each oxygen atom, with six valence electrons, needs two more electrons. We distribute the remaining valence electrons to form double bonds between the phosphorus and oxygen atoms:
- P
- ---O
- |
- |
- O=O
Step 5: Formal Charge Calculation
To ensure the stability of the molecule, we calculate the formal charge of each atom. The formal charge helps us determine if the current electron distribution is optimal. For phosphorus:
Formal Charge (P) = Valence Electrons - Non-Bonding Electrons - (Bonding Electrons / 2) Formal Charge (P) = 5 - 0 - (6 / 2) = 2
For oxygen atoms:
Formal Charge (O) = Valence Electrons - Non-Bonding Electrons - (Bonding Electrons / 2) Formal Charge (O) = 6 - 4 - (2 / 2) = 0
Since the formal charges are minimized and the octets are satisfied, we have achieved a stable Lewis structure for phosphate.
Understanding the Phosphate Lewis Structure

The phosphate Lewis structure provides us with valuable insights into the behavior and properties of this important compound:
- Electron Distribution: The Lewis structure visually represents how electrons are distributed among the atoms. This distribution influences the molecule’s reactivity and chemical properties.
- Bonding Pattern: The double bonds between phosphorus and oxygen atoms indicate a stronger bond compared to single bonds. This bonding pattern affects the molecule’s stability and reactivity.
- Octet Rule Satisfaction: The octet rule, satisfied in the phosphate Lewis structure, ensures that each atom has a stable electron configuration. This stability contributes to the molecule’s overall stability.
- Negative Charge: The overall negative charge of the phosphate ion (-3) is distributed evenly among the oxygen atoms. This charge influences the molecule’s behavior in ionic compounds and its interaction with other ions.
Applications of Phosphate Lewis Structure
Understanding the Lewis structure of phosphate has practical implications in various fields:
- Biochemistry: Phosphate plays a crucial role in biological processes, including DNA and RNA structure, energy transfer, and cellular signaling. The Lewis structure helps researchers understand phosphate’s behavior in these complex systems.
- Environmental Science: Phosphate compounds are essential in soil fertility and aquatic ecosystems. The Lewis structure aids in studying phosphate’s role in nutrient cycles and its impact on environmental health.
- Chemical Synthesis: Phosphate esters and salts are widely used in chemical synthesis, pharmaceuticals, and industrial processes. The Lewis structure provides insights into the reactivity and behavior of these compounds.
- Analytical Chemistry: Phosphate is a common analyte in various analytical techniques. Understanding its Lewis structure is crucial for accurate identification and quantification in analytical chemistry.
Expert Perspective: Dr. Emily Thompson, Organic Chemist
“The Lewis structure of phosphate is a fundamental tool in our understanding of its chemical behavior. By visualizing the electron distribution and bonding patterns, we can predict and explain its reactivity, making it an invaluable asset in various research and industrial applications.”
Case Study: Phosphate in DNA Structure

Phosphate’s role in DNA structure is a fascinating application of its Lewis structure. DNA, the carrier of genetic information, consists of a sugar-phosphate backbone and nitrogenous bases. The phosphate groups, linked to the sugar molecules, provide stability and structural integrity to the DNA molecule. The Lewis structure of phosphate helps researchers understand how these negatively charged groups interact with the positively charged sugar molecules, forming the double helix structure of DNA.
Future Trends: Phosphate in Green Chemistry
Phosphate compounds are increasingly being explored in the field of green chemistry. As sustainability becomes a priority, phosphate-based materials and processes offer promising alternatives. The Lewis structure of phosphate aids researchers in designing and optimizing these sustainable solutions, ensuring their stability and efficiency.
Key Takeaways
- The Lewis structure of phosphate provides a visual representation of its electron distribution and bonding patterns.
- Phosphate’s role in various fields, from biochemistry to environmental science, is closely tied to its Lewis structure.
- Understanding the Lewis structure helps predict and explain phosphate’s reactivity and behavior.
- Researchers and scientists leverage the Lewis structure to optimize phosphate-based applications in sustainable and innovative ways.
Frequently Asked Questions (FAQs)
How does the Lewis structure of phosphate affect its reactivity in chemical reactions?
+The Lewis structure of phosphate, with its double bonds and negative charge, influences its reactivity. The double bonds indicate stronger bonds, making phosphate less reactive than compounds with single bonds. The negative charge also affects its interaction with other ions, impacting its reactivity in ionic compounds.
Can the Lewis structure of phosphate be used to predict its behavior in biological systems?
+Absolutely! The Lewis structure of phosphate provides valuable insights into its behavior in biological systems. It helps researchers understand how phosphate interacts with other molecules, such as DNA and proteins, and how it contributes to essential biological processes like energy transfer and cellular signaling.
What are some common applications of phosphate compounds in industrial processes?
+Phosphate compounds find diverse applications in industrial processes. They are used in the production of fertilizers, detergents, flame retardants, and pharmaceuticals. The Lewis structure of phosphate aids in designing and optimizing these processes, ensuring their efficiency and stability.
How does the negative charge of phosphate affect its behavior in ionic compounds?
+The negative charge of phosphate plays a crucial role in its behavior in ionic compounds. It interacts with positively charged ions, forming stable ionic bonds. This charge distribution influences the overall stability and reactivity of the ionic compound, impacting its physical and chemical properties.
What are the environmental implications of phosphate compounds in aquatic ecosystems?
+Phosphate compounds, when released into aquatic ecosystems, can have significant environmental impacts. Excessive phosphate levels can lead to eutrophication, causing algal blooms and disrupting the balance of aquatic ecosystems. Understanding the Lewis structure of phosphate helps researchers study its behavior and develop strategies to mitigate these environmental concerns.
In conclusion, the phosphate Lewis structure is a powerful tool that offers valuable insights into the behavior and properties of this essential compound. From its role in biological processes to its applications in sustainable chemistry, understanding the Lewis structure of phosphate is crucial for researchers, scientists, and professionals across various fields.



