The Complex World of Water's Phase Diagram
Water, a seemingly simple and ubiquitous substance, holds within it a complex and fascinating phase diagram that has captivated scientists for centuries. This intricate map of water’s various states and transitions offers a profound insight into the fundamental nature of matter and the behavior of one of Earth’s most essential elements. Delving into this diagram reveals a tapestry of phase changes, each with its unique characteristics and implications, that not only informs our understanding of water but also sheds light on the broader principles governing the physical world.
In this article, we embark on a journey through the depths of water’s phase diagram, unraveling its intricacies, exploring the underlying physics, and uncovering the practical applications and real-world implications of water’s remarkable behavior. From the everyday phenomena we witness to the cutting-edge technologies it enables, water’s phase diagram serves as a testament to the wonders of scientific exploration and the endless mysteries of the natural world.
The Fundamental Nature of Water’s States
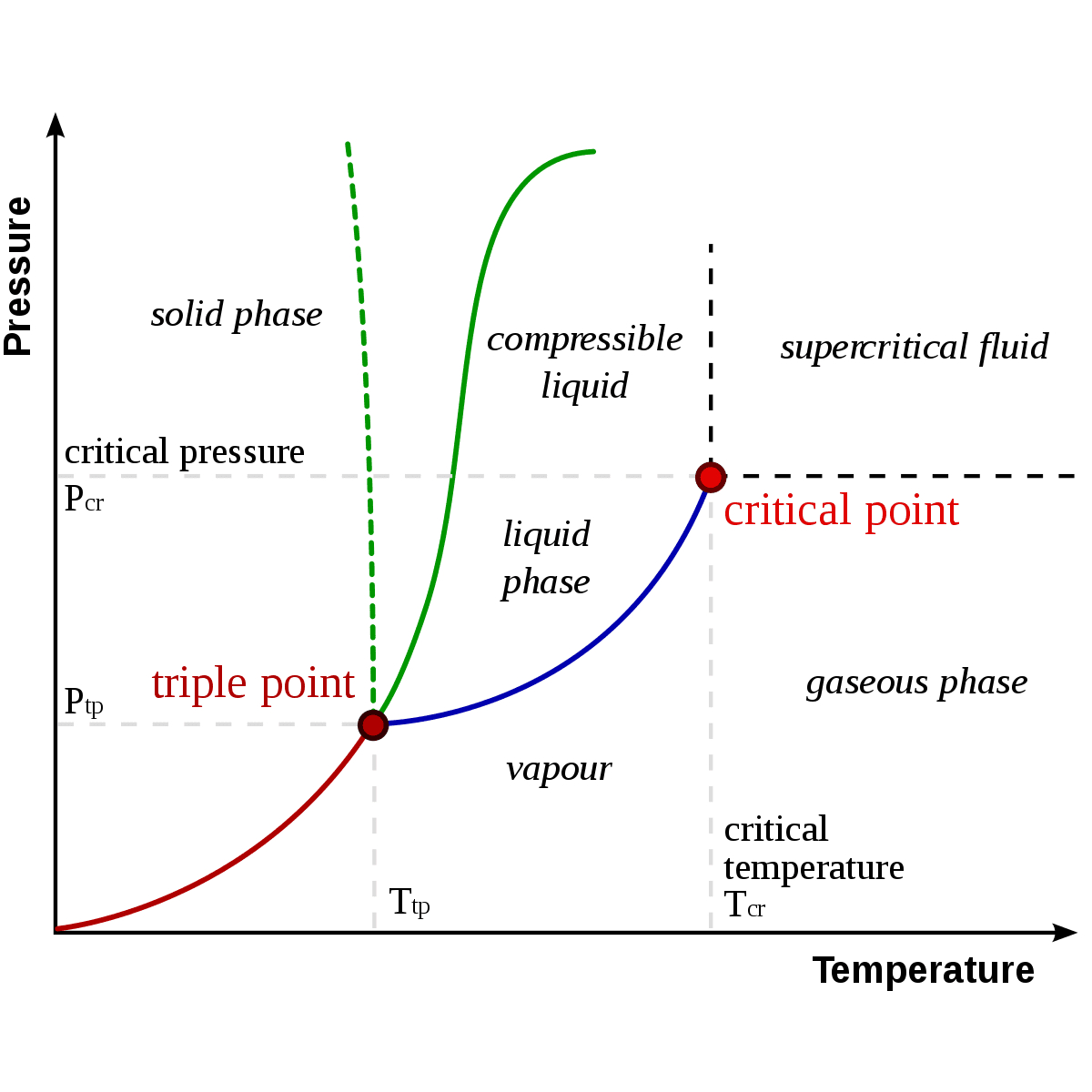
At its core, water’s phase diagram depicts the various states that water can exist in under different conditions of temperature and pressure. These states, often referred to as phases, include the well-known solid, liquid, and gas phases, but also encompass less familiar states like supercritical fluids and exotic forms like amorphous ice. Each of these phases represents a unique arrangement of water molecules, with distinct properties and behaviors that have profound implications across scientific disciplines and practical applications.
The diagram itself is a complex interplay of thermodynamic principles, with phase transitions occurring at critical points where the energy balance between molecular forces and external conditions shifts. These transitions, which can be as dramatic as the freezing of a lake or as subtle as the gradual densification of water under pressure, are governed by intricate molecular interactions and quantum mechanical effects.
Exploring the Key Phases and Transitions
One of the most fundamental and well-known transitions in water’s phase diagram is the freezing point, where liquid water transforms into solid ice. This transition, which occurs at 0°C (32°F) under standard atmospheric pressure, is a result of the hydrogen bonding between water molecules, causing them to arrange into a crystalline lattice structure. The resulting ice has a lower density than liquid water, leading to the peculiar phenomenon of ice floating on water—a critical factor in Earth’s climate and ecosystems.
Moving further along the diagram, we encounter the boiling point, where liquid water transitions into water vapor. This transition, which occurs at 100°C (212°F) under standard conditions, is a result of the thermal energy overcoming the intermolecular forces, allowing water molecules to escape into the gas phase. The boiling point is a key parameter in many industrial processes, from cooking to power generation, and understanding its nuances is critical for efficient and safe operations.
The Enigmatic World of Supercritical Fluids
Beyond the familiar states of solid, liquid, and gas, water’s phase diagram reveals a fascinating realm of supercritical fluids. These are unique states that occur at conditions where both temperature and pressure exceed the critical point, a threshold beyond which the distinction between liquid and gas becomes blurred. In this realm, water behaves as a dense, highly compressible fluid with properties that can be tuned by adjusting the temperature and pressure.
Supercritical water has a range of intriguing properties, including the ability to dissolve a wide variety of substances, making it a powerful solvent in industrial processes and a promising medium for extracting valuable compounds from biomass. It also exhibits unique transport properties, with enhanced mass and heat transfer rates, which have potential applications in energy storage and conversion systems.
Amorphous Ice and the Quantum World
Diving deeper into the complexities of water’s phase diagram, we encounter the enigmatic world of amorphous ice. Unlike the crystalline structure of regular ice, amorphous ice lacks long-range order, resulting in a unique, non-crystalline structure. This form of ice is produced under specific conditions of rapid cooling or high pressure, and it exhibits intriguing properties that have captivated scientists for decades.
Amorphous ice is of particular interest in the field of quantum computing, where its unique molecular arrangement and low-temperature properties make it a promising medium for quantum information storage and processing. Researchers are exploring ways to harness the peculiar quantum behavior of water molecules in amorphous ice to develop new technologies that could revolutionize computing and communications.
Real-World Applications and Implications
The insights gained from studying water’s phase diagram have profound implications across a range of fields and applications. In meteorology and climate science, a deep understanding of water’s phase transitions is critical for modeling weather patterns, predicting extreme events, and assessing the impacts of climate change. The behavior of water in its various phases directly influences atmospheric circulation, cloud formation, and precipitation patterns, shaping the very fabric of Earth’s climate system.
In the realm of materials science and nanotechnology, water’s phase diagram informs the development of new materials and technologies. For example, understanding the behavior of water at extreme pressures and temperatures has led to the creation of novel materials with unique properties, such as high-temperature superconductors and advanced ceramics. These materials have applications in everything from energy storage and transmission to aerospace and medical technologies.
Navigating the Complexities: A Practical Guide
For those navigating the complexities of water’s phase diagram in practical applications, a range of tools and techniques are available to aid in understanding and predicting phase transitions. Computational modeling, for example, allows scientists and engineers to simulate the behavior of water under different conditions, providing valuable insights into the molecular dynamics and thermodynamics of phase changes.
Experimental techniques, such as calorimetry and differential scanning calorimetry, offer direct measurements of heat flow and phase transitions, providing critical data for validating models and informing practical applications. These tools, combined with a deep understanding of the underlying physics, enable researchers and engineers to optimize processes, develop new technologies, and make informed decisions in a wide range of industries and scientific disciplines.
The Ever-Expanding Horizons of Water Science
As our understanding of water’s phase diagram deepens, so too does our appreciation for the vastness and complexity of water science. From the fundamental physics of molecular interactions to the cutting-edge applications in quantum computing and nanotechnology, water continues to reveal its secrets and inspire new avenues of exploration.
The study of water’s phase diagram is not merely an academic pursuit but a gateway to unlocking the potential of one of Earth’s most vital resources. As we continue to explore and harness the unique properties of water, we not only advance scientific knowledge but also develop solutions to some of the world’s most pressing challenges, from sustainable energy to climate resilience.
Conclusion: A Tapestry of Wonder
In conclusion, water’s phase diagram stands as a testament to the intricate beauty and complexity of the natural world. From the familiar states of ice and steam to the exotic realms of supercritical fluids and amorphous ice, water’s phase transitions offer a profound insight into the fundamental nature of matter and the vast possibilities of scientific exploration.
As we navigate the intricacies of water’s phase diagram, we are not just charting the behavior of a single substance but mapping the very fabric of the physical world. In doing so, we not only deepen our understanding of the universe but also unlock the potential to shape a more sustainable, resilient, and technologically advanced future.
Frequently Asked Questions (FAQs)
What is the critical point in water's phase diagram, and why is it significant?
+The critical point in water's phase diagram is the threshold beyond which the distinction between liquid and gas becomes blurred. It occurs at a temperature of approximately 374°C (705°F) and a pressure of 22.1 MPa (3,205 psi). This point is significant because it marks the transition from classical thermodynamics to a regime where quantum effects become dominant, leading to the unique properties of supercritical fluids.
<div class="faq-item">
<div class="faq-question">
<h3>How does the behavior of water in its various phases impact climate and weather patterns?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>The behavior of water in its various phases has a profound impact on climate and weather patterns. For example, the freezing of water into ice at 0°C (32°F) drives the formation of clouds and precipitation, while the boiling point of water at 100°C (212°F) influences atmospheric circulation and convection processes. Understanding these phase transitions is critical for modeling and predicting weather patterns and assessing the impacts of climate change.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What are some practical applications of supercritical water, and how is it harnessed in industry?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Supercritical water has a range of practical applications, including its use as a powerful solvent for extracting valuable compounds from biomass. It is also utilized in energy storage and conversion systems due to its enhanced mass and heat transfer rates. In industry, supercritical water is harnessed through carefully controlled processes that maintain the required temperature and pressure conditions.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>How does the study of amorphous ice contribute to advancements in quantum computing and nanotechnology?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>The study of amorphous ice, with its unique molecular arrangement and low-temperature properties, has the potential to revolutionize quantum computing and nanotechnology. The peculiar quantum behavior of water molecules in amorphous ice makes it a promising medium for quantum information storage and processing, offering the possibility of developing new technologies with unprecedented computational power and data storage capabilities.</p>
</div>
</div>
</div>



