The Periodic Table: Unlocking Molar Mass
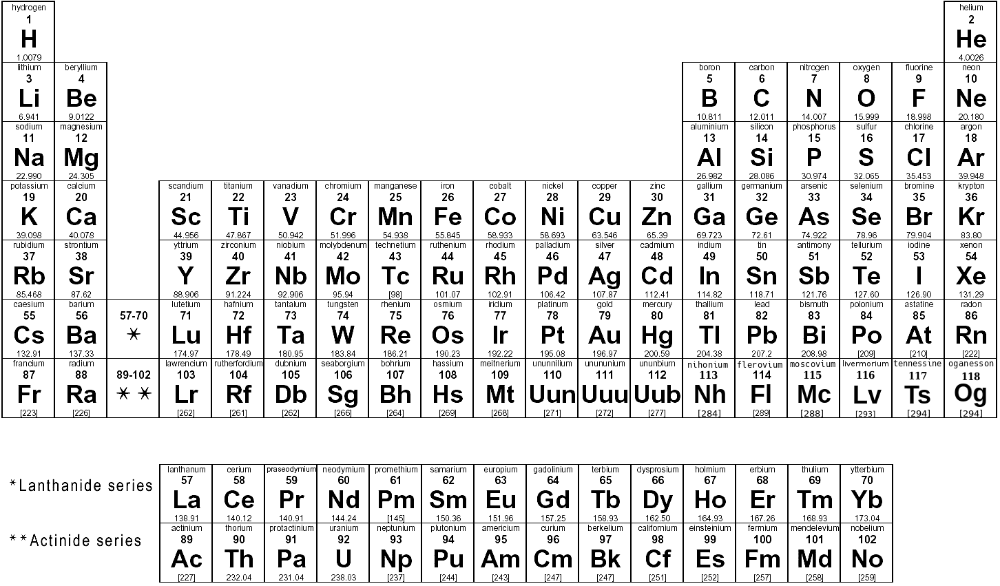
Molar mass, a fundamental concept in chemistry, is the key to understanding the building blocks of our universe. It serves as a bridge between the microscopic world of atoms and the macroscopic realm we inhabit. By delving into the intricacies of the periodic table, we can unlock the secrets of molar mass and explore its significance in various scientific disciplines.
The periodic table is not just a static arrangement of elements; it is a dynamic roadmap that guides us through the vast chemical landscape. Each element, with its unique atomic structure, contributes to the tapestry of molar mass calculations.
Let’s embark on a journey to unravel the mysteries of molar mass and discover how this concept shapes our understanding of matter.
The Building Blocks: Atoms and Their Masses

At the heart of molar mass lies the concept of atoms, the basic units of matter. Every element in the periodic table is composed of atoms, each with its distinct properties and characteristics. To comprehend molar mass, we must first explore the atomic realm.
Step 1: Atomic Structure
Atoms consist of three primary subatomic particles: protons, neutrons, and electrons. Protons and neutrons, collectively known as nucleons, reside in the atom's nucleus, while electrons orbit around it in discrete energy levels or shells.
The number of protons in an atom's nucleus defines its atomic number, which determines the element's identity. For instance, carbon, with an atomic number of 6, has six protons in its nucleus.
Step 2: Atomic Mass
The mass of an atom is primarily determined by the number of nucleons (protons and neutrons) it contains. While protons and neutrons have nearly identical masses, electrons contribute negligibly to the overall mass.
Atomic mass is measured in atomic mass units (amu) or unified atomic mass units (u). One atomic mass unit is approximately equal to the mass of one proton or neutron.
Unlocking Molar Mass: A Molecular Perspective
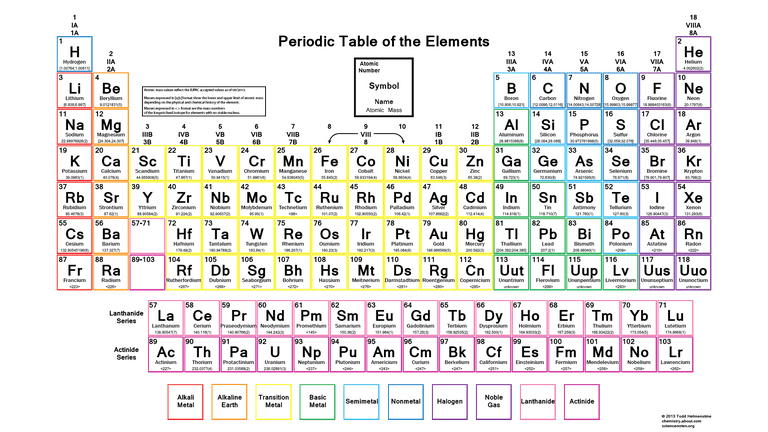
Molar mass takes us beyond the realm of individual atoms and introduces us to the concept of molecules, which are combinations of atoms. Molecules are the building blocks of compounds, and their molar mass is a critical factor in various chemical processes.
Molar mass, expressed in grams per mole (g/mol), represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, containing 6.022 x 10^23 particles, known as Avogadro's number.
Pros of Understanding Molar Mass:
- Precise chemical calculations: Molar mass enables accurate measurements and stoichiometric calculations, ensuring the success of chemical reactions.
- Molecular weight determination: It helps identify the molecular weight of compounds, aiding in the characterization and identification of substances.
Cons of Molar Mass:
- Complexity for beginners: Molar mass calculations can be daunting for newcomers to chemistry, requiring a solid understanding of atomic and molecular concepts.
- Potential for errors: Mistakes in molar mass calculations can lead to inaccurate results and failed experiments, emphasizing the need for precision.
Calculating Molar Mass: A Step-by-Step Guide
Calculating molar mass is a fundamental skill for chemists and scientists. Let’s break down the process into simple steps:
Step 1: Identify the Substance
Begin by determining the substance for which you want to calculate the molar mass. This could be an element, a molecule, or a compound.
Step 2: Determine Atomic Masses
Consult the periodic table to find the atomic masses of the elements involved. Atomic masses are typically listed below each element's symbol.
Step 3: Calculate Molar Mass
For elements, the molar mass is simply the atomic mass. For molecules or compounds, multiply the atomic mass of each element by the number of atoms it contains in the molecule or compound.
Let's take water (H2O) as an example. Hydrogen has an atomic mass of approximately 1 u, and oxygen has an atomic mass of approximately 16 u. To calculate the molar mass of water:
- 2 x Atomic mass of Hydrogen (1 u) = 2 u
- 1 x Atomic mass of Oxygen (16 u) = 16 u
- Total molar mass of water = 2 u + 16 u = 18 u
Thus, the molar mass of water is approximately 18 g/mol.
Real-World Applications: Molar Mass in Action
Molar mass finds extensive applications across various scientific fields, showcasing its practical relevance:
Pharmaceuticals:
- Drug formulation: Molar mass calculations are crucial for determining precise dosages and ensuring therapeutic effectiveness.
- Pharmaceutical analysis: It aids in identifying unknown substances and characterizing drug compounds.
Environmental Science:
- Air pollution monitoring: Molar mass is used to quantify the concentration of pollutants, aiding in environmental assessments.
- Water quality analysis: It helps determine the composition of water samples, identifying contaminants and their concentrations.
Materials Science:
- Polymer synthesis: Molar mass calculations guide the production of polymers with specific properties, ensuring quality control.
- Nanomaterial research: Molar mass plays a vital role in characterizing nanomaterials, contributing to advancements in nanotechnology.
Future Trends: Molar Mass in Emerging Technologies

As scientific advancements continue to shape our world, molar mass remains a cornerstone concept with far-reaching implications:
Quantum Computing:
The development of quantum computers relies on precise control of atomic and molecular systems. Molar mass calculations will play a critical role in designing and optimizing quantum algorithms.
Genomics and Bioinformatics:
In the era of genomic research, molar mass calculations are essential for understanding the molecular basis of life. They contribute to the analysis of DNA, RNA, and protein sequences.
Sustainable Energy:
The transition to sustainable energy sources, such as hydrogen fuel cells, depends on accurate molar mass calculations for optimizing fuel efficiency and minimizing environmental impact.
Unlocking the Full Potential: Molar Mass Education
Empowering future generations with a solid understanding of molar mass is crucial for advancing scientific progress. Educators play a vital role in making this concept accessible and engaging:
Interactive Simulations:
Utilize interactive simulations and virtual laboratories to bring molar mass calculations to life. Visual representations enhance student engagement and comprehension.
Real-World Connections:
Connect molar mass concepts to everyday scenarios, such as cooking, pharmaceuticals, or environmental issues. Relating theory to practice deepens students' understanding.
Collaborative Projects:
Encourage collaborative projects that apply molar mass calculations to real-world problems. This fosters critical thinking, teamwork, and a deeper appreciation for the subject.
In conclusion, the periodic table holds the key to unlocking the mysteries of molar mass. By exploring the atomic and molecular realms, we gain a profound understanding of matter and its properties. Molar mass calculations are not just theoretical concepts but powerful tools with practical applications across diverse scientific disciplines. As we continue to advance our knowledge, molar mass will remain a cornerstone of scientific exploration and innovation.
How does molar mass differ from molecular weight?
+Molar mass and molecular weight are closely related concepts but have subtle differences. Molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol). Molecular weight, on the other hand, refers to the sum of the atomic masses of all atoms in a molecule and is typically expressed in atomic mass units (amu) or unified atomic mass units (u). While molar mass is specific to a substance, molecular weight applies to individual molecules.
<div class="faq-item">
<div class="faq-question">
<h3>Can molar mass be used to identify unknown substances?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Yes, molar mass calculations can aid in the identification of unknown substances. By determining the molar mass of a sample, scientists can compare it to known compounds or use it as a basis for further analysis, such as mass spectrometry or chromatography, to identify the substance.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>How does molar mass impact chemical reactions?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Molar mass is crucial for understanding and predicting chemical reactions. It enables chemists to calculate the quantities of reactants and products involved in a reaction, ensuring stoichiometric balance. Molar mass calculations also help determine the limiting reactant, which is essential for optimizing reaction efficiency and yield.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Are there any limitations to molar mass calculations?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>While molar mass calculations are highly accurate for pure substances, they may encounter challenges when dealing with complex mixtures or compounds with variable compositions. In such cases, additional analytical techniques, such as chromatography or spectroscopy, are employed to obtain more precise results.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>How has the concept of molar mass evolved over time?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>The concept of molar mass has evolved alongside the development of modern chemistry. Initially, chemists relied on empirical methods to determine atomic and molecular masses. With the discovery of Avogadro's number and the development of accurate measuring instruments, molar mass calculations became more precise and reliable, contributing to our current understanding of matter.</p>
</div>
</div>
</div>



