The Periodic Table: 5 Orbital Insights
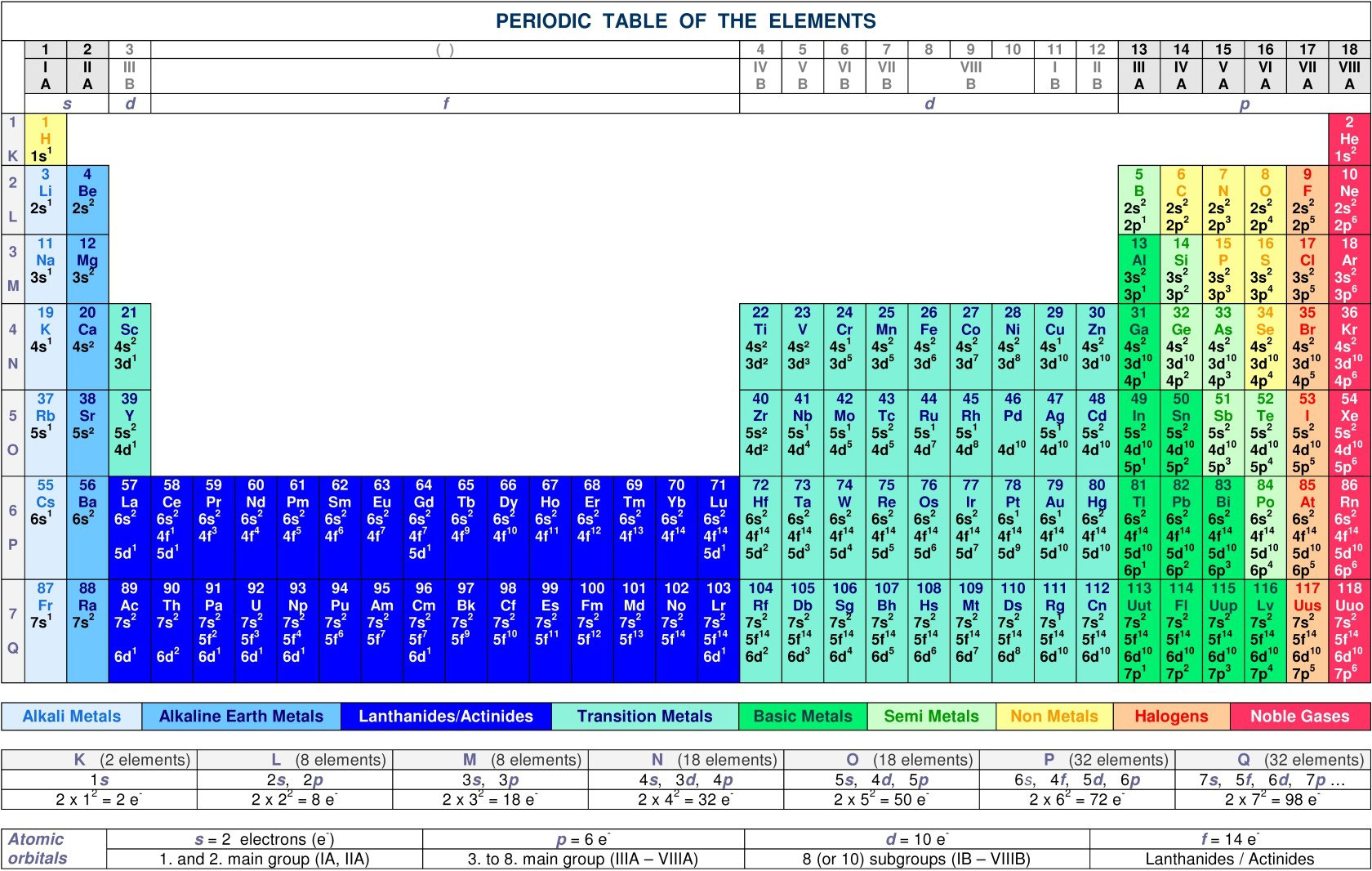
Unveiling the Complexity of Atomic Orbitals

In the vast expanse of scientific discovery, the Periodic Table stands as a cornerstone, offering a systematic understanding of the elements that comprise our universe. Yet, beneath its orderly columns and rows lie intricate intricacies, notably the concept of atomic orbitals. These orbitals, far from static shells, represent the intricate dance of electrons around atomic nuclei, defining the very essence of chemical behavior.
This article delves into five profound insights derived from these orbital intricacies, each unearthing a layer of complexity that adds to our understanding of the Periodic Table and, by extension, the natural world.

The Evolution of Orbital Theory
The journey towards comprehending atomic orbitals is a testament to scientific progression. From the pioneering work of physicists like Bohr, whose model envisioned electrons as occupying discrete energy levels, to the quantum mechanical advancements of Schrödinger and Heisenberg, our understanding has evolved significantly.
The modern conception of orbitals, as described by quantum mechanics, portrays them as regions of space where electrons are most likely to be found. These regions are not rigid, but rather probabilistic, reflecting the wave-like nature of electrons. This shift from a classical, deterministic view to a quantum, probabilistic one is a paradigm shift that underpins much of modern chemistry.
Orbital Shapes and Symmetry

One of the most visually captivating aspects of atomic orbitals is their unique shapes and symmetries. The familiar s, p, d, and f orbitals, each with their distinct geometries, offer a glimpse into the complexity of electron distribution.
- S-orbitals, for instance, are spherical, indicating a uniform probability of finding an electron in any direction from the nucleus.
- P-orbitals, with their dumbbell shapes, suggest a directional preference, aligning with the axes of the atom.
- D and f-orbitals, with their more intricate shapes, play crucial roles in the chemistry of transition and inner-transition metals, influencing their unique properties and behaviors.
Understanding these shapes is not merely academic; it has practical implications in fields like spectroscopy, where the unique energy transitions associated with specific orbital configurations provide a means to identify and characterize chemical substances.
The Role of Orbitals in Chemical Bonding
Orbitals are not merely abstract concepts; they are the very foundation of chemical bonding. When atoms combine to form molecules, it is the interaction and overlap of their orbitals that dictate the nature and strength of these bonds.
In covalent bonds, electrons are shared between atoms, often resulting in the formation of new, molecular orbitals that encompass the entire molecule. These molecular orbitals, like their atomic counterparts, have unique shapes and energy levels, influencing the molecule's stability, reactivity, and physical properties.
Moreover, the concept of hybridization, where atomic orbitals combine to form new, hybrid orbitals, is a testament to the dynamic nature of orbitals. This process, observed in molecules like methane (CH4), where the carbon's 2s and 2p orbitals hybridize to form four equivalent sp3 hybrid orbitals, is a fundamental aspect of organic chemistry.
Orbital Filling and the Periodic Table
The arrangement of elements in the Periodic Table is, in large part, a consequence of orbital filling. As electrons are added to an atom, they fill the lowest energy orbitals first, following the Aufbau principle.
This process of orbital filling, however, is not as straightforward as it might seem. The order in which orbitals are filled is dictated by the Madelung rule, which accounts for the complex interplay between the principal quantum number (n), the azimuthal quantum number (l), and the number of electrons in the atom.
The resulting pattern of orbital filling is a key factor in determining an element's chemical properties. For instance, the presence of half-filled or fully-filled orbital subshells can lead to increased stability, influencing an element's reactivity and its position in the Periodic Table.
Exploring the Frontiers of Orbital Theory
While our understanding of atomic orbitals has advanced significantly, there are still frontiers to explore. For instance, the precise nature of the transition between classical and quantum mechanics remains a topic of active research, with implications for our understanding of fundamental physics.
Additionally, the study of relativistic effects on orbitals, particularly for elements in the later periods of the Periodic Table, presents a complex challenge. These effects, while relatively minor for lighter elements, become significant for heavier elements, influencing their chemical behavior in subtle yet profound ways.
In exploring the intricacies of atomic orbitals, we not only deepen our understanding of the Periodic Table but also gain insights into the very fabric of our universe. From the evolution of orbital theory to the practical implications for chemical bonding, these five insights offer a glimpse into the complexity and beauty of the atomic world.
Conclusion
As we navigate the intricacies of atomic orbitals, we are reminded of the ever-evolving nature of scientific knowledge. The Periodic Table, while a testament to our understanding, also serves as a roadmap to the unknown, guiding us towards new discoveries and a deeper comprehension of the world around us.
By unraveling the mysteries of atomic orbitals, we not only enhance our grasp of chemical behavior but also contribute to the broader narrative of scientific exploration. In this journey, each insight, each new understanding, is a step towards a more profound appreciation of the universe's underlying principles.
How do atomic orbitals influence chemical bonding?
+Atomic orbitals play a crucial role in chemical bonding by dictating the nature and strength of bonds. When atoms combine, their orbitals overlap, forming new molecular orbitals that encompass the entire molecule. The shape and energy levels of these molecular orbitals influence the molecule’s stability, reactivity, and physical properties.
What is the significance of orbital shapes and symmetries?
+Orbital shapes and symmetries are visually captivating and provide insights into the complexity of electron distribution. S-orbitals are spherical, p-orbitals are dumbbell-shaped, and d and f-orbitals have more intricate shapes. These shapes have practical implications, particularly in spectroscopy, where they help identify and characterize chemical substances.
How does the filling of orbitals influence the Periodic Table?
+The arrangement of elements in the Periodic Table is largely determined by orbital filling. Electrons fill the lowest energy orbitals first, following the Aufbau principle and the Madelung rule. This filling pattern influences an element’s chemical properties, with half-filled or fully-filled orbital subshells leading to increased stability and influencing reactivity.
What are some future directions in orbital theory research?
+Future research in orbital theory focuses on exploring the transition between classical and quantum mechanics and studying relativistic effects on orbitals, particularly for heavier elements. These areas of study have implications for fundamental physics and a deeper understanding of chemical behavior.



