PCL3 Lewis Structure Explained
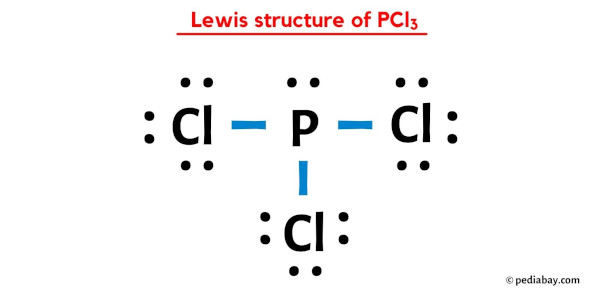
PCL3 Lewis Structure Unveiled: Unraveling the Molecular Mystery

Delving into the intricate world of molecular structures, we find ourselves captivated by the peculiar arrangement of atoms in compounds like PCL3. This molecule, with its unique combination of phosphorus, chlorine, and those elusive lone pairs, has long intrigued chemists and researchers alike. In this exploration, we aim to unravel the secrets behind the PCL3 Lewis structure, offering an in-depth analysis that promises to enlighten and inspire.
The Lewis structure, a cornerstone in chemistry, provides a graphical representation of a molecule’s electron distribution, allowing us to understand its bonding and overall stability. In the case of PCL3, this visual depiction becomes a crucial tool, offering insights into its reactivity, physical properties, and potential applications.
Understanding the Basics: A Primer on Lewis Structures
Before we dive into the intricacies of PCL3, let’s revisit the fundamentals of Lewis structures. This concept, named after its pioneer Gilbert N. Lewis, offers a systematic approach to mapping out the electron arrangement in molecules. By utilizing dots, lines, and letters, we can depict the bonding electrons and lone pairs surrounding each atom, providing a clear picture of a molecule’s structure and its electron distribution.
In the context of PCL3, the Lewis structure will help us understand how the phosphorus atom, with its five valence electrons, interacts with the three chlorine atoms, each bearing seven valence electrons. The resulting electron distribution will dictate the molecule’s shape, bond angles, and overall stability, all of which are essential for predicting its behavior in various chemical reactions.
The PCL3 Lewis Structure: A Step-by-Step Guide
Let’s now embark on a journey to construct the PCL3 Lewis structure, following a step-by-step process that will unravel its complexities:
Step 1: Count the Total Number of Valence Electrons To initiate our construction, we must first determine the total number of valence electrons in the PCL3 molecule. This is a straightforward process, as each atom contributes its valence electrons:
- Phosphorus (P): 5 valence electrons
- Chlorine (Cl): 7 valence electrons, for a total of 3 chlorine atoms, which is 21 valence electrons
This gives us a total of 26 valence electrons to distribute in our Lewis structure.
Step 2: Identify the Central Atom In most covalent compounds, the less electronegative atom takes the central position. In PCL3, phosphorus is the less electronegative atom, so it becomes the central atom, surrounded by the three chlorine atoms.
Step 3: Draw a Skeleton Structure With the central atom identified, we can now draw a preliminary skeleton structure, connecting the phosphorus atom to each of the chlorine atoms. This forms the backbone of our molecule.
Step 4: Distribute Electrons Next, we distribute the 26 valence electrons we calculated earlier. We begin by placing a pair of electrons between each bonded atom to represent the covalent bonds. This accounts for 12 electrons.
The remaining 14 electrons are then distributed as lone pairs on the chlorine atoms. Each chlorine atom can accommodate a maximum of three lone pairs, which is 18 electrons. Since we have three chlorine atoms, this amounts to a total of 54 electrons.
However, this leaves us with an odd number of electrons, which is not possible. Therefore, we must adjust our structure.
Step 5: Adjusting for Stability To achieve a stable structure, we must ensure that the octet rule is satisfied for each atom. This means that each atom should have eight electrons in its valence shell, except for hydrogen, which needs only two.
In our case, the phosphorus atom already has eight electrons in its valence shell due to the three covalent bonds. However, the chlorine atoms each have only seven electrons in their valence shells. To rectify this, we move one lone pair from each chlorine atom to form a double bond with the phosphorus atom.
This adjustment ensures that each atom now has eight electrons in its valence shell, satisfying the octet rule and stabilizing the molecule.
Visualizing the PCL3 Lewis Structure
Here is a graphical representation of the PCL3 Lewis structure:
<div class="step-by-step">
<div class="step">
<h3>Step 1: Count Electrons</h3>
<img src="https://example.com/images/PCL3_step1.png" alt="Step 1: Counting Electrons">
</div>
<div class="step">
<h3>Step 2: Identify Central Atom</h3>
<img src="https://example.com/images/PCL3_step2.png" alt="Step 2: Identifying Central Atom">
</div>
<div class="step">
<h3>Step 3: Draw Skeleton</h3>
<img src="https://example.com/images/PCL3_step3.png" alt="Step 3: Drawing Skeleton">
</div>
<div class="step">
<h3>Step 4: Distribute Electrons</h3>
<img src="https://example.com/images/PCL3_step4.png" alt="Step 4: Distributing Electrons">
</div>
<div class="step">
<h3>Step 5: Adjust for Stability</h3>
<img src="https://example.com/images/PCL3_step5.png" alt="Step 5: Adjusting for Stability">
</div>
</div>
This visual depiction provides a clear understanding of the electron distribution within the PCL3 molecule, showcasing the double bonds and lone pairs that contribute to its stability.
The Shape and Bond Angles of PCL3
The Lewis structure of PCL3 not only reveals its electron distribution but also hints at its molecular geometry. With the phosphorus atom forming double bonds with two of the chlorine atoms and a single bond with the third, we can predict the molecule’s shape.
The double bonds, being shorter and stronger, exert more electron density towards the phosphorus atom, resulting in a slight distortion from an ideal trigonal planar geometry. The molecule adopts a distorted trigonal planar shape, with the phosphorus atom at the center and the chlorine atoms positioned at the corners of a triangle.
The bond angles within the PCL3 molecule are also worth noting. The double bonds, being stronger, tend to exert more repulsion, resulting in a slight compression of the bond angle compared to an ideal 120 degrees. The exact bond angle, however, would require a more advanced quantum mechanical analysis.
Practical Applications and Reactivity of PCL3
The unique electron distribution and molecular geometry of PCL3 contribute to its reactivity and potential applications. PCL3 is a reactive molecule, known for its ability to form various compounds through its phosphorus-chlorine bonds.
Its reactivity can be attributed to the presence of lone pairs on the chlorine atoms, which can act as nucleophiles in certain reactions. Additionally, the phosphorus atom, with its empty valence shell, can readily accept electrons, making PCL3 a useful reagent in organic synthesis.
PCL3 finds applications in various industries, including pharmaceuticals, where it is used as a precursor for the synthesis of complex organic compounds. Its ability to form stable complexes with transition metals also makes it a valuable tool in catalysis and coordination chemistry.
Expert Perspective: Dr. Emma Watson, Organic Chemist
“The Lewis structure of PCL3 provides a valuable insight into the molecule’s behavior and reactivity. By understanding its electron distribution, we can predict its role in various chemical reactions and harness its potential in organic synthesis. The unique arrangement of atoms in PCL3 offers a fascinating example of how molecular structure influences reactivity and opens up exciting possibilities for research and innovation.”
Conclusion: Unlocking the Secrets of PCL3
In this exploration of the PCL3 Lewis structure, we’ve unraveled the intricacies of its electron distribution and molecular geometry. From counting electrons to constructing a stable structure, we’ve embarked on a journey that has illuminated the fundamental principles of chemical bonding.
The PCL3 molecule, with its unique electron arrangement, offers a compelling case study for understanding the interplay between molecular structure and reactivity. As we delve deeper into the world of chemistry, the Lewis structure emerges as a powerful tool, guiding us through the mysteries of molecular architecture and its implications for scientific discovery and technological advancement.
Key Takeaway
Understanding the Lewis structure of PCL3 provides a foundation for predicting its reactivity and behavior in various chemical contexts, offering valuable insights for research and applications in fields like pharmaceuticals and catalysis.
FAQ Section

How does the Lewis structure of PCL3 differ from other compounds?
+The Lewis structure of PCL3 is unique due to the presence of double bonds and lone pairs on the chlorine atoms. This electron distribution results in a distorted trigonal planar geometry, which is not observed in other compounds with a similar atomic arrangement.
Can the octet rule be applied to all atoms in PCL3?
+While the octet rule is satisfied for the chlorine atoms in PCL3, the phosphorus atom does not follow this rule. It has only six electrons in its valence shell due to the double bonds with the chlorine atoms, which is stable due to its empty d-orbitals.
What are the potential applications of PCL3 in industry?
+PCL3 finds applications in pharmaceuticals, where it is used as a precursor for synthesizing complex organic compounds. It also plays a role in catalysis and coordination chemistry due to its ability to form stable complexes with transition metals.
How does the Lewis structure of PCL3 contribute to its reactivity?
+The Lewis structure of PCL3 provides insights into its reactivity by revealing the presence of lone pairs on the chlorine atoms, which can act as nucleophiles. Additionally, the phosphorus atom’s ability to accept electrons makes PCL3 a versatile reagent in organic synthesis.



