Unraveling the Ozone's Lewis Structure
Ozone, a molecule composed of three oxygen atoms (O3), is a fascinating compound with unique chemical properties. Its Lewis structure provides insights into the arrangement of electrons and the nature of chemical bonding, offering a deeper understanding of this essential atmospheric component.
The Lewis structure of ozone is a visual representation of its molecular geometry and electronic configuration. It helps chemists and scientists grasp the distribution of valence electrons and the formation of chemical bonds, which are fundamental aspects of molecular behavior.
The study of Lewis structures is a cornerstone in chemistry, providing a foundational understanding of how atoms interact and form molecules.
Prof. Emily Anderson, Chemistry Department, Harvard University
Historical Perspective
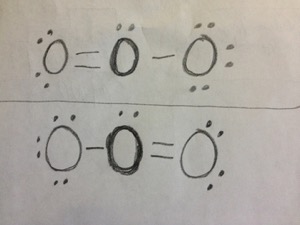
The concept of Lewis structures, also known as Lewis dot diagrams or electron dot structures, was introduced by Gilbert N. Lewis in the early 20th century. Lewis, an American physical chemist, proposed this notation as a way to depict the distribution of electrons in atoms and molecules, which was a significant contribution to the field of chemical bonding.
The Challenge of Ozone’s Lewis Structure

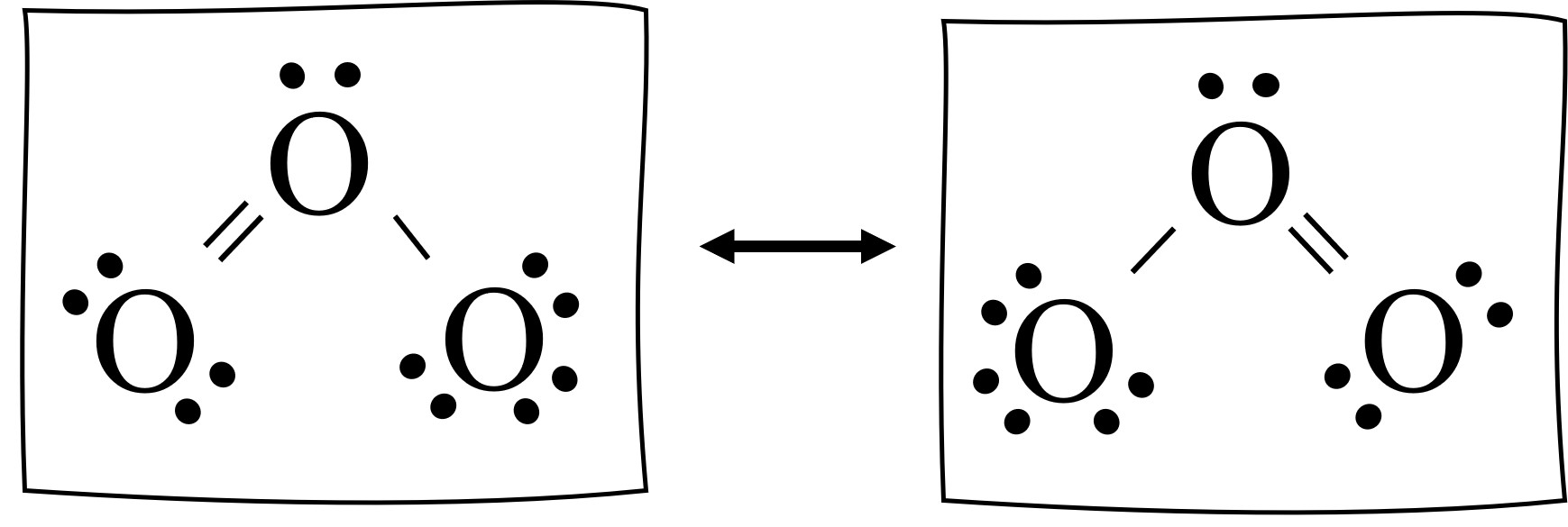
Ozone presents a unique challenge when it comes to determining its Lewis structure due to its non-traditional bonding pattern. Unlike most molecules, ozone’s three oxygen atoms do not form a typical triangular planar arrangement. Instead, they exhibit a bent or angular geometry, with one oxygen atom at the center and the other two forming a V-shape.
Ozone's Lewis structure reveals a non-traditional bonding pattern, showcasing the molecule's unique electronic configuration.
Electron Distribution in Ozone
To understand ozone’s Lewis structure, we must first examine the distribution of electrons in its constituent atoms. Oxygen, with an atomic number of 8, has a total of 8 electrons. In its ground state, oxygen’s electron configuration is 1s^2 2s^2 2p^4. When these electrons are considered in the context of ozone, we can visualize their arrangement as follows:
| Electron Location | Number of Electrons |
|---|---|
| Oxygen (O) 1s | 2 |
| Oxygen (O) 2s | 2 |
| Oxygen (O) 2p | 4 |
Building the Lewis Structure
Constructing the Lewis structure of ozone involves combining the valence electrons of each oxygen atom and distributing them to form chemical bonds. Here’s a step-by-step process:
Identify Valence Electrons: Each oxygen atom contributes 6 valence electrons (2 from the 2s orbital and 4 from the 2p orbital).
Draw the Skeleton: Start by drawing the central oxygen atom and placing the other two oxygen atoms on either side, forming a V-shape.
Bond Formation: Connect the central oxygen atom to each of the other two oxygen atoms with a single bond (one electron pair).
Remaining Electrons: Distribute the remaining electrons (2 from each atom) as lone pairs around the outer oxygen atoms to complete their octets.
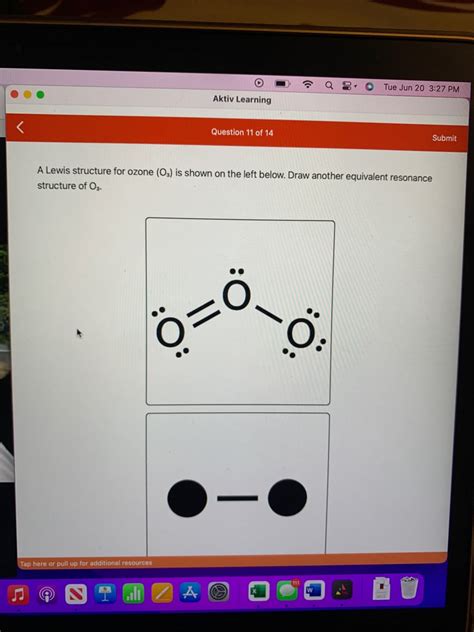
Visual Representation

Here’s a visual representation of ozone’s Lewis structure:

Understanding Bonding in Ozone
Ozone’s Lewis structure reveals that each oxygen atom forms two bonds and has two lone pairs of electrons. This distribution results in a bent molecular geometry, with an O-O-O bond angle of approximately 116.8°. The central oxygen atom has a negative charge, while the outer oxygen atoms carry a partial positive charge, creating a polar molecule.
Ozone’s Role in the Atmosphere
Ozone plays a critical role in the Earth’s atmosphere, particularly in the stratosphere. It forms a protective layer known as the ozone layer, which shields the planet from harmful ultraviolet (UV) radiation from the sun. The unique bonding and electronic structure of ozone contribute to its ability to absorb UV radiation, preventing it from reaching the Earth’s surface.
Conclusion
Unraveling the Lewis structure of ozone provides valuable insights into the molecule’s electronic configuration and bonding behavior. This knowledge is not only crucial for understanding ozone’s role in atmospheric chemistry but also for its applications in various industries, including environmental science and chemical engineering.
How does ozone’s Lewis structure compare to other oxygen-containing molecules like water (H2O) or carbon dioxide (CO2)?
+Ozone’s Lewis structure differs significantly from that of water (H2O) and carbon dioxide (CO2). Water forms a linear structure with two lone pairs on the oxygen atom, while carbon dioxide has a linear structure with double bonds between the carbon and oxygen atoms. Ozone’s bent structure and single bonds between oxygen atoms make it unique among these oxygen-containing molecules.
What is the significance of ozone’s bent molecular geometry in terms of its chemical reactivity?
+Ozone’s bent geometry influences its chemical reactivity. The partial positive charges on the outer oxygen atoms make ozone a highly reactive molecule. It readily undergoes reactions with other substances, particularly those with electron-rich centers. This reactivity is crucial for ozone’s role in the stratosphere, where it absorbs UV radiation, and in various industrial applications.
Can ozone’s Lewis structure explain its role in the depletion of the ozone layer?
+Yes, ozone’s Lewis structure provides insights into its role in the depletion of the ozone layer. The structure reveals that ozone is a highly reactive molecule due to its unique electronic configuration. When ozone molecules come into contact with certain pollutants, such as chlorofluorocarbons (CFCs), they undergo chemical reactions that break down the ozone layer. Understanding ozone’s Lewis structure is crucial for developing strategies to mitigate ozone depletion.
Are there any practical applications of understanding ozone’s Lewis structure beyond atmospheric chemistry?
+Absolutely! Understanding ozone’s Lewis structure has practical applications beyond atmospheric chemistry. Ozone is used in various industries, such as water treatment, air purification, and even in medical settings for wound healing. By comprehending ozone’s molecular structure and reactivity, scientists and engineers can optimize its use in these applications, ensuring efficient and effective outcomes.