What Is the Molar Mass of NH3?
Ammonia, with its chemical formula NH3, is a colorless gas that plays a crucial role in various industries and natural processes. Understanding its molecular composition is essential for chemists, engineers, and environmental scientists alike. The molar mass of NH3 provides valuable insights into its behavior and applications. In this comprehensive exploration, we delve into the intricacies of ammonia’s molecular weight, shedding light on its significance and practical implications.
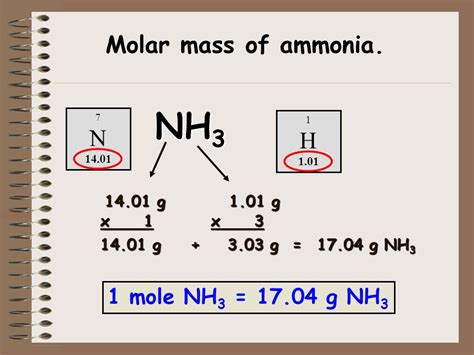
The molar mass of a substance is a fundamental property that indicates the weight of one mole of that substance. It is derived by summing the atomic weights of each element present in the molecule. In the case of ammonia, we have nitrogen (N) and hydrogen (H) atoms. Nitrogen, with an atomic weight of approximately 14.007 amu (atomic mass units), contributes significantly to the overall mass. Each hydrogen atom, with an atomic weight of approximately 1.008 amu, adds a small fraction to the total.
To calculate the molar mass of NH3, we simply add the atomic weights of nitrogen and three hydrogen atoms:
Molar Mass of NH3 = Atomic Weight of Nitrogen + (Atomic Weight of Hydrogen x 3)
Molar Mass of NH3 = 14.007 amu + (1.008 amu x 3) Molar Mass of NH3 = 14.007 amu + 3.024 amu Molar Mass of NH3 = 17.031 amu
Therefore, the molar mass of ammonia is approximately 17.031 amu. This value is a critical parameter in various scientific and industrial applications, influencing reaction kinetics, stoichiometry, and gas laws.
Ammonia’s molar mass is a testament to the precision with which chemical elements combine to form compounds. This fundamental property is a cornerstone for understanding ammonia’s behavior, whether in the context of its role as a fertilizer, a refrigerant, or a key player in atmospheric chemistry.
The molar mass of NH3, at approximately 17.031 amu, is a fundamental characteristic that influences ammonia's behavior and applications across diverse fields.
Pros of Ammonia's Molar Mass
- It facilitates precise stoichiometric calculations in chemical reactions.
- Molar mass is crucial for determining the concentration of ammonia solutions, a common parameter in various industries.
- This property aids in understanding gas laws and their application in ammonia-based systems.
Cons and Limitations
- Ammonia's molar mass is relatively small, which can make it challenging to handle in some processes that require precise mass measurements.
- The simplicity of ammonia's molecular structure and low molar mass may limit its potential for certain complex applications.
How is molar mass calculated for compounds like NH3?
+Molar mass is calculated by summing the atomic weights of each element in the compound. For NH3, you add the atomic weight of nitrogen (N) and three hydrogen (H) atoms. The formula is: Molar Mass = Atomic Weight of Nitrogen + (Atomic Weight of Hydrogen x 3)
<div class="faq-item">
<div class="faq-question">
<h3>What are the implications of ammonia's low molar mass in industrial processes?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Ammonia's low molar mass can make it challenging to handle in some processes, especially those that require precise mass measurements. However, its low mass also contributes to its high reactivity and versatility, making it a valuable compound in various industries.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Can the molar mass of NH3 be determined experimentally, or is it a theoretical value?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>The molar mass of NH3 is primarily a theoretical value derived from the atomic weights of nitrogen and hydrogen. While it can be experimentally confirmed through various analytical techniques, the theoretical calculation is generally accurate and widely accepted.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What role does ammonia's molar mass play in its environmental impact?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Ammonia's molar mass is indirectly related to its environmental impact. Its low molar mass contributes to its high volatility and ease of dispersion in the atmosphere, which can lead to environmental concerns such as air pollution and ecological imbalances.</p>
</div>
</div>
</div>



