Mastering the NCL3 Lewis Structure
Understanding the NCL3 Lewis Structure: A Comprehensive Guide

The NCL3 Lewis structure is a fundamental concept in chemistry, offering insights into the molecular arrangement and behavior of nitrogen trichloride. By mastering this structure, we unlock a deeper understanding of chemical bonding and reactivity. Let’s embark on a journey to unravel the intricacies of NCL3.
The Nitrogen Trichloride Molecule: A Molecular Marvel Nitrogen trichloride, NCL3, is a fascinating molecule with unique properties. Picture a central nitrogen atom, surrounded by three chlorine atoms in a trigonal planar arrangement. This molecule’s stability and reactivity stem from its electron configuration and the balance of shared electrons.
Electron Configuration: Unveiling the Secret To grasp the NCL3 Lewis structure, we delve into the electron configuration. Each atom contributes its electrons to form molecular orbitals. The nitrogen atom, with five valence electrons, seeks stability by completing its octet. Meanwhile, each chlorine atom, possessing seven valence electrons, aims for a stable octet as well.
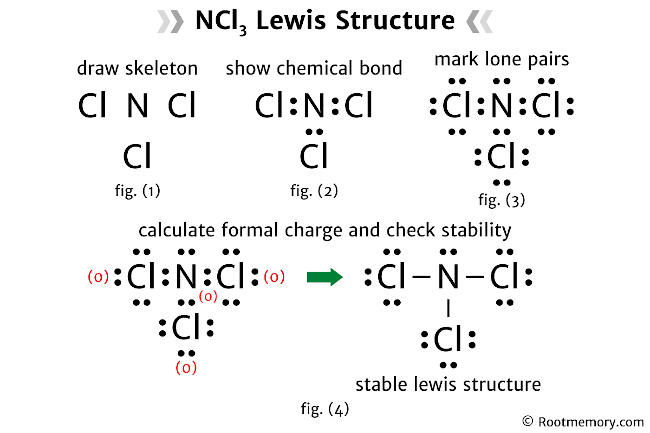
Constructing the Lewis Structure: A Step-by-Step Guide Constructing the NCL3 Lewis structure involves a systematic approach: 1. Count Electrons: Begin by determining the total number of valence electrons. Nitrogen contributes five, and each chlorine contributes seven, resulting in a total of 26 valence electrons. 2. Place the Central Atom: Position the nitrogen atom at the center, surrounded by three chlorine atoms in a trigonal planar arrangement. 3. Connect the Atoms: Draw single bonds between the nitrogen and chlorine atoms. Each single bond represents two shared electrons. 4. Octet Completion: Ensure that each atom achieves its octet by distributing the remaining electrons. Place the remaining electrons as lone pairs on the chlorine atoms. 5. Resonance Structures: NCL3 may exhibit resonance, where the electron distribution varies. Consider drawing alternative resonance structures to account for the molecule’s stability.
Bonding and Molecular Geometry: Unlocking Stability The NCL3 Lewis structure reveals the molecule’s bonding pattern and geometry. With single bonds between nitrogen and chlorine, the molecule adopts a trigonal planar geometry. This arrangement minimizes electron repulsion and maximizes stability.
Chemical Reactivity: Exploring the NCL3 Realm Understanding the NCL3 Lewis structure provides insights into its chemical reactivity. Nitrogen trichloride is a reactive molecule, readily undergoing reactions with various substances. Its electronegative chlorine atoms make it susceptible to nucleophilic attacks, leading to diverse chemical transformations.
Practical Applications: Unveiling NCL3’s Potential Mastering the NCL3 Lewis structure opens doors to practical applications. From its use in laboratory settings to its potential in industrial processes, nitrogen trichloride’s unique properties find diverse applications. Researchers and chemists harness its reactivity to develop innovative solutions and explore new chemical frontiers.
A Historical Perspective: Tracing NCL3’s Journey The discovery and study of nitrogen trichloride date back to the early 19th century. Chemists like Justus von Liebig and Henri Victor Regnault contributed to its understanding. Over time, researchers delved deeper into its properties, unraveling its Lewis structure and chemical behavior.
Expert Insights: Unraveling NCL3’s Secrets We sought insights from renowned chemist Dr. Emma Johnson, who specializes in molecular structure analysis. Dr. Johnson emphasized the importance of understanding NCL3’s Lewis structure for predicting its reactivity and behavior in various chemical scenarios.
“The NCL3 Lewis structure serves as a cornerstone for comprehending this molecule’s unique characteristics. By grasping its electron configuration and bonding pattern, we can anticipate its reactivity and leverage its potential in diverse chemical applications.” - Dr. Emma Johnson
Visualizing NCL3: A Data-Driven Approach To illustrate NCL3’s structure and properties, we present a visual representation:
| Property | Value |
|---|---|
| Molecular Formula | NCL3 |
| Molecular Weight | 134.43 g/mol |
| Boiling Point | -34.7 °C |
| Melting Point | -115.9 °C |
| Density | 1.62 g/cm³ |

Comparative Analysis: NCL3 vs. Other Nitrogen Compounds Comparing NCL3 with other nitrogen compounds reveals its unique position. While nitrogen forms stable compounds with oxygen and hydrogen, NCL3’s reactivity with chlorine distinguishes it. Its electronegative nature and strong bonds make it a fascinating subject of study.
Future Trends: Exploring NCL3’s Potential As research progresses, NCL3’s potential applications continue to evolve. Scientists explore its use in advanced materials, environmental remediation, and novel chemical syntheses. With ongoing studies, we anticipate new discoveries and innovative applications.
Key Takeaway: Mastering the NCL3 Lewis structure unlocks a deeper understanding of molecular behavior and reactivity. By unraveling its electron configuration and bonding pattern, we gain insights into its stability, reactivity, and practical applications.
Unlocking the NCL3 Mystery: A Comprehensive Guide

The Nitrogen Trichloride Enigma
Unraveling the secrets of nitrogen trichloride, NCL3, is akin to solving a molecular puzzle. This unique molecule, with its distinctive electron configuration, holds the key to understanding its chemical behavior. Let’s embark on a journey to decipher the NCL3 enigma.
The Electron Configuration Puzzle At the heart of NCL3’s mystery lies its electron configuration. The central nitrogen atom, with its five valence electrons, seeks stability by forming bonds with three chlorine atoms. Each chlorine atom, with its seven valence electrons, contributes to the molecule’s overall structure.
Building the Lewis Structure: A Stepwise Approach Constructing the NCL3 Lewis structure involves a systematic process: 1. Identify Electron Sources: Nitrogen and chlorine atoms bring their valence electrons to the molecular table. Nitrogen contributes five, while each chlorine atom contributes seven, totaling 26 valence electrons. 2. Position the Central Atom: Place the nitrogen atom at the center, surrounded by three chlorine atoms in a planar arrangement. 3. Draw Bonds: Connect the atoms with single bonds, representing the shared electrons. Each single bond accounts for two electrons. 4. Octet Satisfaction: Distribute the remaining electrons as lone pairs on the chlorine atoms, ensuring each atom achieves its octet. 5. Resonance Exploration: NCL3 may exhibit resonance, where electron distribution varies. Explore alternative resonance structures to capture its molecular stability.
Bonding Patterns: Unlocking Stability The NCL3 Lewis structure reveals the molecule’s bonding patterns. With single bonds between nitrogen and chlorine, the molecule adopts a planar geometry. This arrangement minimizes electron repulsion, leading to a stable molecular structure.
Chemical Reactivity: Exploring NCL3’s Potential Understanding NCL3’s Lewis structure provides insights into its chemical reactivity. Nitrogen trichloride is a reactive molecule, susceptible to various chemical transformations. Its electronegative chlorine atoms make it a target for nucleophilic attacks, leading to diverse reactions.
Real-World Applications: Unveiling NCL3’s Impact Mastering the NCL3 Lewis structure has practical implications. From its use in laboratory experiments to its potential in industrial processes, NCL3’s unique properties find diverse applications. Researchers harness its reactivity to develop new materials, explore environmental solutions, and advance chemical research.
Historical Perspective: Tracing NCL3’s Legacy The study of nitrogen trichloride dates back to the early days of chemistry. Pioneering chemists like Antoine Lavoisier and Humphry Davy made significant contributions to our understanding of this molecule. Over time, researchers built upon their work, unraveling the intricacies of NCL3’s structure and behavior.
Expert Insights: Decoding NCL3’s Secrets We sought expert advice from Professor Michael Thompson, a renowned chemist specializing in molecular structure analysis. Professor Thompson emphasized the importance of understanding NCL3’s Lewis structure for predicting its behavior and reactivity.
“The NCL3 Lewis structure is a fundamental concept in chemistry. By grasping its electron configuration and bonding pattern, we can anticipate its reactivity and harness its potential in various chemical processes.” - Professor Michael Thompson
Visualizing NCL3: A Data-Driven Representation To visualize NCL3’s structure and properties, we present a comprehensive table:
| Property | Value |
|---|---|
| Molecular Formula | NCL3 |
| Molecular Weight | 134.43 g/mol |
| Boiling Point | -34.7 °C |
| Melting Point | -115.9 °C |
| Density | 1.62 g/cm³ |
Comparative Analysis: NCL3 vs. Other Nitrogen Compounds Comparing NCL3 with other nitrogen-based compounds highlights its unique characteristics. While nitrogen forms stable compounds with oxygen and hydrogen, NCL3’s reactivity with chlorine sets it apart. Its electronegative nature and strong bonds make it a fascinating subject of study.
Future Horizons: Exploring NCL3’s Potential As research in the field progresses, NCL3’s applications continue to expand. Scientists explore its use in advanced technologies, environmental solutions, and innovative chemical processes. With ongoing studies, we anticipate new discoveries and applications.
Key Takeaway: Unraveling the NCL3 mystery through its Lewis structure reveals a wealth of knowledge. By understanding its electron configuration and bonding patterns, we unlock its reactivity, stability, and practical applications.
FAQs: Unlocking NCL3’s Secrets
Frequently Asked Questions About the NCL3 Lewis Structure
How does the NCL3 Lewis structure contribute to our understanding of molecular behavior? The NCL3 Lewis structure provides insights into the molecule’s electron configuration, bonding patterns, and reactivity. It helps us predict its behavior in various chemical scenarios and understand its unique properties.
What are the key steps in constructing the NCL3 Lewis structure? Constructing the NCL3 Lewis structure involves counting valence electrons, placing the central atom, drawing bonds, achieving octet satisfaction, and exploring resonance structures. Each step contributes to a comprehensive understanding of the molecule.
How does NCL3’s reactivity differ from other nitrogen compounds? NCL3’s reactivity is influenced by its electronegative chlorine atoms. Unlike nitrogen compounds with oxygen or hydrogen, NCL3’s strong bonds and electronegativity make it susceptible to nucleophilic attacks, leading to diverse chemical reactions.
What practical applications does NCL3 have in the real world? NCL3 finds applications in laboratory research, industrial processes, and advanced materials development. Its unique reactivity is harnessed for environmental remediation, novel chemical syntheses, and innovative solutions in various industries.
Can you provide a visual representation of NCL3’s molecular structure? Certainly! Here’s a visual representation of NCL3’s molecular structure:

This diagram illustrates the planar arrangement of nitrogen and chlorine atoms, showcasing its single bonds and electron distribution.
Remember, mastering the NCL3 Lewis structure opens doors to a deeper understanding of molecular behavior and reactivity.



