A New Take on Bohr's Model
Historical Evolution of Bohr’s Model
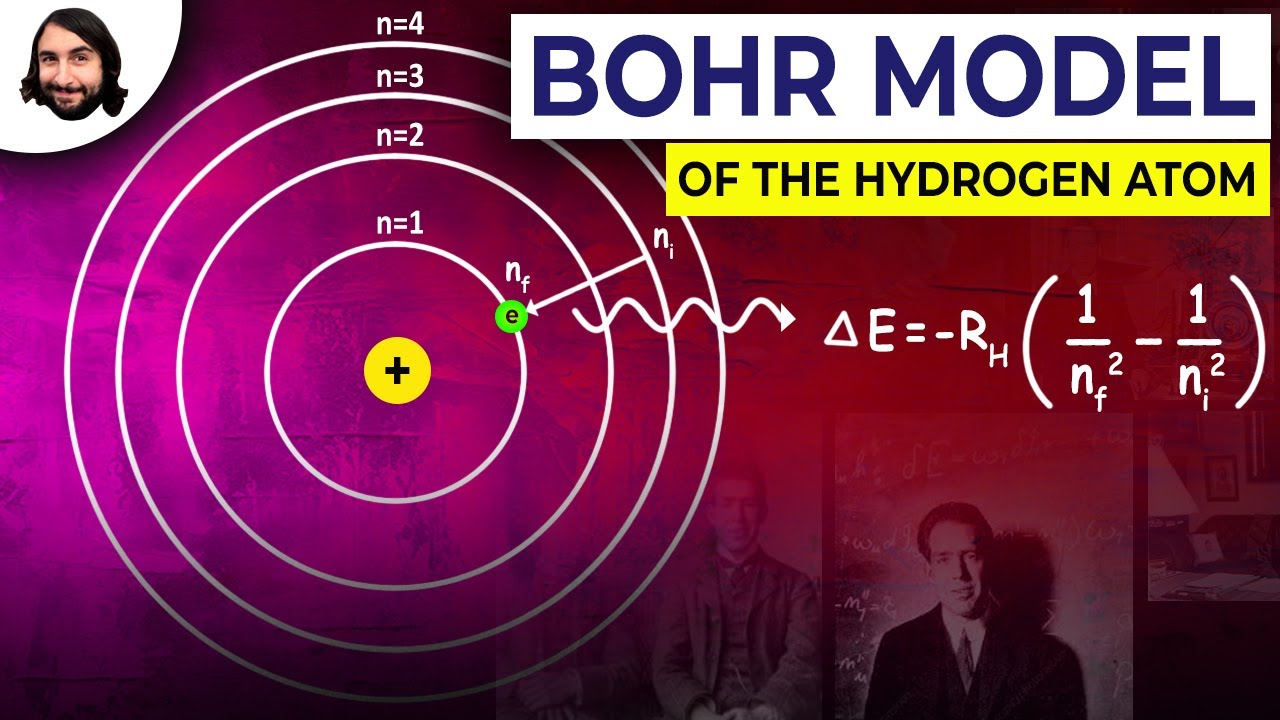
The origins of the Bohr model can be traced back to the early 20th century, a period of intense scientific exploration and groundbreaking discoveries. It was a time when the foundations of quantum mechanics were being laid, challenging the classical understanding of physics. Bohr, a Danish physicist, introduced his model in 1913, aiming to explain the spectral lines of hydrogen atoms. His model was a bold departure from the prevailing classical physics, incorporating quantum principles to explain the behavior of electrons within atoms.
Bohr’s model proposed that electrons orbit the nucleus in discrete energy levels or shells. This was a radical idea, as it suggested that electrons could only exist in specific, quantized energy states. This concept, known as quantization, was a game-changer, as it explained the discrete spectral lines observed in atomic spectra. Each energy level corresponded to a specific energy, and as electrons transitioned between these levels, they emitted or absorbed energy in the form of photons, giving rise to the distinct spectral lines.
Modern Interpretations and Adaptations

While the Bohr model provided a groundbreaking framework, it was soon recognized that it had limitations when applied to more complex atoms and phenomena. The development of quantum mechanics led to the emergence of more sophisticated models, such as the Schrödinger wave equation, which offered a probabilistic description of electron behavior.
However, the Bohr model’s simplicity and intuitive appeal have ensured its continued relevance and adaptation in various contexts. Here’s how modern interpretations have evolved:
Quantum Mechanics Integration: Modern physics incorporates Bohr’s ideas within the broader framework of quantum mechanics. While the Schrödinger equation provides a more accurate description, the Bohr model’s concept of energy levels persists, offering a simplified representation that is particularly useful in introductory physics courses.
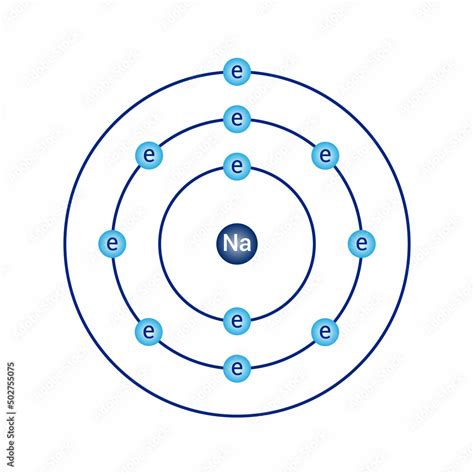
Orbital Visualization: In modern chemistry, the Bohr model is often employed to visualize electron configurations and orbital filling. Although more advanced models like the electron cloud model provide a more precise representation, the Bohr model’s discrete energy levels offer a straightforward way to understand electron distribution.
Atomic Spectroscopy: The spectral lines observed in atomic spectroscopy are still explained using Bohr’s principles. When electrons transition between energy levels, they emit or absorb specific frequencies of light, leading to the characteristic emission and absorption spectra. This phenomenon is fundamental in fields like astronomy and analytical chemistry.
Exploring the Nuances
Beyond its core principles, the Bohr model also provides a foundation for exploring intriguing atomic phenomena:
Electron Spin: While Bohr’s model primarily focused on electron energy levels, modern physics introduces the concept of electron spin. Spin is an intrinsic property of electrons, adding another dimension to their behavior. This aspect is crucial in understanding phenomena like magnetism and the behavior of particles in spintronics.
Atomic Radii and Size: The Bohr model’s energy levels are closely tied to the concept of atomic radii. As electrons occupy higher energy levels, they tend to be farther from the nucleus, leading to larger atomic radii. This relationship is essential in understanding the periodic trends observed in the periodic table.
Electron Transitions and Excitation: The model’s energy level concept explains how electrons can be excited to higher energy levels when absorbing energy. This process is fundamental in understanding fluorescence, phosphorescence, and the behavior of electrons in lasers and optical devices.
Practical Applications and Real-World Impact
The Bohr model’s impact extends beyond the theoretical realm, finding practical applications in various fields:
Spectroscopy and Astronomy: The understanding of spectral lines, as explained by Bohr’s model, is vital in astronomy. By analyzing the spectral lines of distant stars and galaxies, astronomers can determine their chemical composition and physical conditions.
Quantum Computing: While quantum computing relies on more advanced quantum principles, the concept of energy levels and transitions, as introduced by Bohr, forms a foundational basis. Quantum bits, or qubits, utilize the principles of superposition and entanglement, which are rooted in the quantization of energy levels.
Atomic Clocks and Precision Timing: Atomic clocks, which are among the most precise timekeeping devices, operate based on the principle of electron transitions between energy levels. By measuring the frequency of these transitions, highly accurate time standards are established, with applications in satellite navigation and fundamental physics research.
Looking Ahead: Future Trends and Emerging Research

As our understanding of atomic physics continues to evolve, researchers are exploring new frontiers and pushing the boundaries of our knowledge. Here’s a glimpse into some of the emerging trends and research areas:
Quantum Information Processing: The field of quantum information science is advancing rapidly, with researchers developing quantum algorithms and protocols that leverage the principles of superposition and entanglement. This has implications for secure communication, optimization problems, and quantum simulations.
Attosecond Physics: With the development of advanced laser technologies, researchers are now capable of generating attosecond pulses of light. This enables the study of atomic and molecular processes at incredibly short timescales, opening up new avenues for understanding electron dynamics and ultrafast phenomena.
Quantum Materials: The exploration of novel materials, such as topological insulators and quantum spin liquids, is a thriving area of research. These materials exhibit unique electronic and magnetic properties, offering potential applications in quantum computing, spintronics, and energy storage.
Final Thoughts
Niels Bohr’s model, despite its simplicity, continues to be a cornerstone of our atomic understanding. Its historical significance, modern adaptations, and practical applications underscore its enduring value. As we delve deeper into the quantum realm, the Bohr model serves as a foundational stepping stone, inspiring further exploration and discovery.
The evolution of atomic theory is a testament to the dynamic nature of scientific knowledge, where initial insights are refined, expanded, and reimagined. As we navigate the intricate tapestry of atomic phenomena, the Bohr model remains a guiding light, illuminating the path toward a deeper comprehension of the quantum world.



