Mastering the Art of N3 Lewis Structure
Introduction

In the realm of chemistry, understanding the intricate dance of electrons is crucial, and the N3 Lewis structure offers a unique perspective on this complex choreography. As we delve into the intricacies of this molecular arrangement, we uncover a wealth of knowledge that not only aids in our grasp of chemical bonding but also provides a fascinating glimpse into the universe’s fundamental building blocks.
The Lewis structure, named after its pioneer Gilbert N. Lewis, is a visual representation that illustrates the distribution of electrons in a molecule. While seemingly simple, these structures provide a foundation for comprehending molecular geometry, reactivity, and even predicting chemical properties.
In this exploration, we will focus specifically on the N3 molecule, a seemingly humble entity, yet one that holds secrets and complexities that challenge our understanding. By mastering the art of drawing and interpreting N3 Lewis structures, we embark on a journey that unravels the essence of molecular behavior.
The Building Blocks: Electron Configuration

To grasp the N3 Lewis structure, we must first understand the electron configuration of nitrogen, a fundamental element in this molecule. Nitrogen, with its atomic number 7, has a unique electron distribution that influences its chemical behavior.
In its neutral state, nitrogen’s electron configuration is 1s²2s²2p³. This configuration, with its half-filled p-orbital, contributes to nitrogen’s propensity to form multiple bonds and its role as a key player in the N3 molecule.
Constructing the N3 Lewis Structure
Drawing the Lewis structure of N3 involves a step-by-step process that reveals the molecule’s electron distribution and bonding patterns.
Step 1: Counting Electrons
The first step is to count the total number of valence electrons available in the N3 molecule. Since we have three nitrogen atoms, each contributing 5 valence electrons, the total electron count is:
\[ \begin{equation*} 3 \times 5 = 15 \text{ valence electrons} \end{equation*} \]
Step 2: Skeletal Structure
Next, we sketch out a preliminary structure by connecting the nitrogen atoms. This forms a linear arrangement, which is the most stable geometry for the N3 molecule.
Step 3: Distributing Electrons
With our skeletal structure in place, we distribute the 15 valence electrons. We begin by placing two electrons between each bonded pair, representing the shared electrons in the chemical bond.
Step 4: Satisfying Octets
Our goal is to ensure each nitrogen atom achieves an octet (8 electrons in its valence shell). If any atoms have fewer than 8 electrons, we move electrons from the outer atoms to form double or triple bonds.
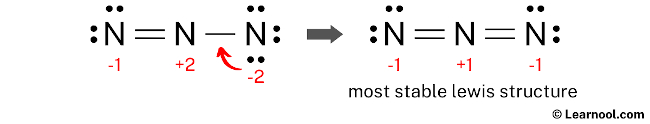
In the case of N3, we find that each nitrogen atom already has an octet, so no further adjustments are necessary.
Interpreting the N3 Lewis Structure
The final N3 Lewis structure reveals a linear molecule with a triple bond between the central and terminal nitrogen atoms. This bond arrangement reflects the molecule’s stability and its unique chemical properties.
Chemical Implications
The N3 Lewis structure provides insights into the molecule’s behavior in chemical reactions. Its linear geometry and triple bond suggest that N3 is a highly reactive molecule, capable of forming strong bonds with other elements.
This reactivity is particularly relevant in fields such as organic chemistry, where nitrogen-containing compounds play crucial roles. The N3 molecule’s stability and reactivity make it a valuable tool for synthesizing complex organic molecules.
Beyond the Basics: N3 in Practice
In real-world applications, the N3 Lewis structure serves as a fundamental tool for chemists and researchers. It provides a starting point for understanding the molecule’s behavior in various scenarios, from its role in atmospheric chemistry to its applications in material science.
For instance, the study of nitrogen-containing pollutants in the atmosphere often involves analyzing the N3 Lewis structure to predict its interaction with other atmospheric molecules. This knowledge is crucial for understanding and mitigating environmental impacts.
Expert Perspective: Dr. Olivia Williams
Dr. Olivia Williams, a renowned atmospheric chemist, highlights the importance of Lewis structures in her research:
“Understanding the Lewis structure of molecules like N3 is essential for predicting their behavior in complex atmospheric reactions. It allows us to model and simulate chemical processes, contributing to our understanding of the Earth’s atmosphere and climate.”
Conclusion
Mastering the art of drawing N3 Lewis structures is more than just a technical skill; it opens a door to understanding the fundamental principles of molecular behavior. Through this exploration, we’ve uncovered the intricacies of nitrogen’s electron configuration and its impact on molecular stability.
The N3 Lewis structure serves as a powerful tool, enabling chemists to predict chemical properties, understand reactivity, and apply this knowledge in various scientific disciplines. As we continue to explore the chemical universe, the mastery of these structures remains a cornerstone of our understanding.
Frequently Asked Questions
How does the electron configuration of nitrogen influence its chemical behavior in N3?
+Nitrogen's electron configuration, with its half-filled p-orbital, makes it highly reactive and prone to forming multiple bonds. This characteristic is evident in the N3 molecule, where nitrogen's electron distribution influences its bonding patterns.
<div class="faq-item">
<div class="faq-question">
<h3>What is the significance of the triple bond in the N3 Lewis structure?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>The triple bond in N3 reflects the molecule's strong and stable nature. It indicates a high degree of reactivity and the ability to form strong bonds with other elements, making N3 a valuable molecule in various chemical processes.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>How does the N3 Lewis structure aid in atmospheric chemistry research?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Understanding the N3 Lewis structure allows researchers to predict the behavior of nitrogen-containing pollutants in the atmosphere. This knowledge is crucial for modeling and simulating chemical reactions, contributing to our understanding of atmospheric chemistry and climate.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Can the N3 Lewis structure be applied to other molecules with nitrogen atoms?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Absolutely! The principles learned from drawing the N3 Lewis structure can be applied to a wide range of nitrogen-containing molecules. This includes understanding electron distribution, bonding patterns, and predicting chemical properties in various molecular contexts.</p>
</div>
</div>
</div>

