Unveiling the Building Blocks: Monomers
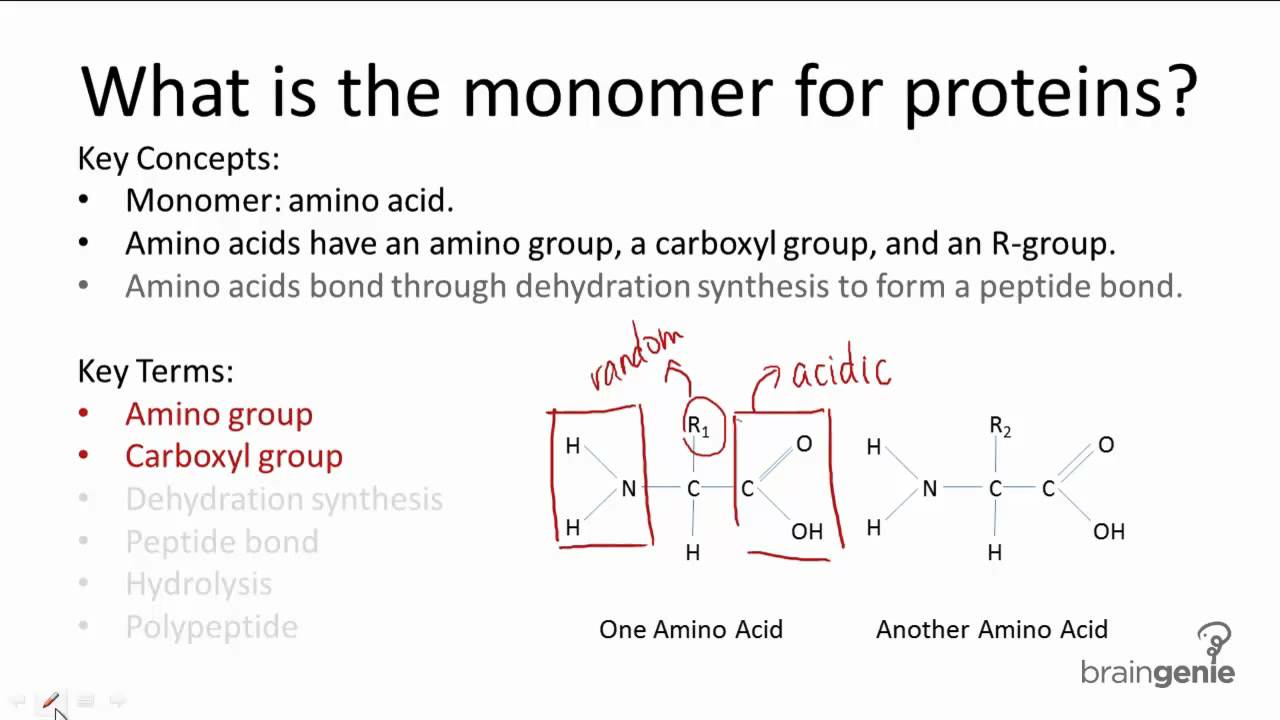
The world of chemistry is an intricate tapestry woven with various components, each playing a crucial role in the grand scheme of things. Among these, monomers stand out as fundamental entities, serving as the basic building blocks for larger, more complex structures. These singular molecules possess the unique ability to form chains, resulting in polymers—a process that underpins much of modern material science. Understanding monomers and their properties is essential for anyone seeking to delve into the fascinating realm of polymer chemistry.
The Monomer Identity Crisis

Monomers often find themselves in a state of identity confusion, as they can be derived from a wide array of chemical families. Organic compounds, specifically hydrocarbons, are the primary source of monomers. These hydrocarbons, in their simplest forms, consist of carbon and hydrogen atoms. However, the addition of other elements, such as oxygen, nitrogen, and chlorine, can lead to the creation of an almost infinite variety of monomers, each with its own distinct properties and potential applications.
Diversity in Monomer Types

The realm of monomers is vast and diverse, with each type offering unique characteristics and behaviors. Some of the most common monomers include:
Ethylene: A simple hydrocarbon, ethylene is the foundation for many polymers, including the ubiquitous polyethylene. Its versatility and ease of processing make it a popular choice in various industries.
Propylene: With an additional carbon atom, propylene forms the basis of polypropylene, a material known for its toughness and resistance to fatigue. Polypropylene finds extensive use in packaging and automotive components.
Styrene: Derived from benzene, styrene is a key component in the production of polystyrene, a material renowned for its clarity and rigidity. Polystyrene’s applications range from food packaging to electronic enclosures.
Vinyl Chloride: This monomer, derived from chlorine and ethylene, is the building block of polyvinyl chloride (PVC), a versatile material with uses spanning from construction to healthcare.
Acrylonitrile: A monomer with a unique structure, acrylonitrile forms the basis of materials like acrylic fibers and certain types of rubber. Its ability to form strong, durable polymers makes it a valuable resource in the textile and automotive industries.
The Polymerization Process
The true magic of monomers lies in their ability to link together, forming long chains known as polymers. This process, called polymerization, can occur through various mechanisms, including addition and condensation reactions.
The resulting polymers can exhibit vastly different properties depending on the monomer structure, the method of polymerization, and the specific conditions under which the reaction occurs.
Applications of Polymers
The products of monomer polymerization find applications in virtually every aspect of modern life. From the plastic bottles we use daily to the high-performance materials in aerospace and automotive industries, polymers derived from monomers are ubiquitous.
Future Prospects

As our understanding of monomers and polymerization deepens, so too does our ability to innovate and create new materials. With ongoing research, we can expect to see the development of monomers with enhanced properties, leading to even more advanced polymers.
Conclusion
Monomers are the unsung heroes of the chemical world, serving as the foundation for the complex and fascinating world of polymers. Their diverse nature and unique properties continue to drive innovation, shaping the materials that underpin our modern world.
What is the main difference between monomers and polymers?
+Monomers are single molecules that can combine to form larger structures called polymers. Polymers are essentially chains or networks of monomers linked together through chemical reactions.
Can all monomers form polymers?
+Not all monomers have the ability to form polymers. The specific chemical structure of a monomer determines whether it can undergo polymerization. Some monomers may require specific conditions or catalysts to initiate the polymerization process.
Are all polymers derived from monomers?
+No, not all polymers are derived from monomers. There are natural polymers, such as cellulose in plants and proteins in our bodies, which are not formed from monomers but are instead biopolymers. Synthetic polymers, on the other hand, are typically derived from monomers through various chemical processes.
How are monomers used in everyday life?
+Monomers are the building blocks of many common materials we interact with daily. For example, ethylene monomers are used to produce polyethylene, which is found in plastic bags, bottles, and various consumer goods. Styrene monomers are used to make polystyrene, a common material in disposable cups and food containers. These are just a few examples of the widespread use of monomers in our daily lives.