Unraveling the Mysteries: Molecular Geometry
The realm of molecular geometry is a captivating one, offering a glimpse into the intricate dance of atoms that forms the foundation of our physical world. This field of study delves into the spatial arrangement of atoms within molecules, revealing the secrets of their structure and behavior. From the simplest diatomic molecules to complex organic compounds, understanding molecular geometry is crucial for chemists, physicists, and biologists alike. Let’s embark on a journey to explore the mysteries that lie within this fascinating realm.
Historical Perspective: A Journey to Unravel the Invisible

The quest to comprehend molecular geometry traces back to the early days of modern chemistry. In the late 19th century, scientists like J.H. van’t Hoff and Joseph Le Bel laid the groundwork for this discipline by proposing the tetrahedral theory. This theory suggested that carbon atoms, with their unique ability to form four bonds, arranged themselves in a tetrahedral shape, thereby influencing the geometry of many organic molecules. This hypothesis, though simple, sparked a revolution in our understanding of molecular structure.
Over the years, advancements in experimental techniques, such as X-ray crystallography and spectroscopy, provided more concrete evidence for the theories proposed. These tools allowed scientists to visualize the three-dimensional structure of molecules, confirming the tetrahedral arrangement of carbon and paving the way for more complex geometric models.
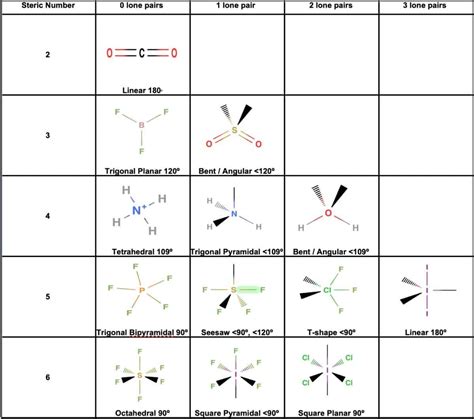
The Language of Molecular Geometry: VSEPR and Beyond

One of the most widely used theories in molecular geometry is the Valence Shell Electron Pair Repulsion (VSEPR) model. Proposed by Dr. Ronald Gillespie and Dr. Ronald Nyholm, this model predicts the geometry of molecules based on the repulsion between electron pairs in the valence shell of an atom. By considering the number of bonding and non-bonding electron pairs, VSEPR theory enables us to predict the geometry of molecules, such as the linear shape of carbon dioxide (CO2) or the trigonal planar arrangement of boron trifluoride (BF3).
However, VSEPR is just the beginning. As our understanding of quantum mechanics and computational chemistry advanced, more sophisticated theories emerged. Quantum mechanical calculations, for instance, allow us to predict and visualize molecular orbitals, providing a deeper insight into the electronic structure and bonding patterns. Additionally, molecular modeling software and computational techniques have become invaluable tools, enabling us to simulate and predict the behavior of molecules under various conditions.
Case Study: Water’s Unique Geometry
Water, a seemingly simple molecule, serves as an excellent example of the complexities of molecular geometry. Comprising just two hydrogen atoms and one oxygen atom, water exhibits a bent molecular geometry with an angle of approximately 104.5 degrees between the hydrogen atoms. This unique geometry is a result of the electronegativity difference between oxygen and hydrogen, leading to the formation of a polar molecule with a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms.
The consequences of water’s geometry are far-reaching. This polar nature gives water its exceptional ability to dissolve a wide range of substances, earning it the title of the “universal solvent.” Furthermore, the hydrogen bonding between water molecules contributes to its high boiling point, making it an essential component of life as we know it.
Future Trends: Unlocking New Possibilities
As we continue to advance in our understanding of molecular geometry, exciting possibilities lie ahead. The development of new experimental techniques, such as high-resolution imaging and advanced spectroscopic methods, promises to provide even more detailed insights into molecular structure. Additionally, the field of computational chemistry is rapidly evolving, with machine learning and artificial intelligence playing a significant role in predicting and simulating molecular behavior.
Furthermore, the integration of molecular geometry with other fields, such as materials science and nanotechnology, opens up new avenues for innovation. By manipulating molecular geometry, scientists can design materials with unique properties, from highly efficient solar cells to advanced medical devices.
Practical Applications: From Pharmaceuticals to Materials Science

The practical applications of molecular geometry are vast and diverse. In the pharmaceutical industry, understanding molecular geometry is crucial for drug design. By manipulating the arrangement of atoms within a molecule, scientists can optimize drug efficacy, minimize side effects, and enhance drug delivery. For instance, the geometry of a drug molecule can influence its solubility, absorption, and interaction with biological targets.
In materials science, molecular geometry plays a pivotal role in designing advanced materials with specific properties. For example, the arrangement of atoms in carbon nanotubes, a prime example of molecular geometry, gives them exceptional strength and electrical conductivity, making them valuable for applications ranging from electronics to aerospace.
Myth vs. Reality: Debunking Common Misconceptions
As with any scientific field, molecular geometry is not without its misconceptions. One common myth is that molecular geometry is solely determined by the number of atoms in a molecule. While this is a factor, it is not the sole determinant. The electronic structure, bond angles, and intermolecular forces also play significant roles in shaping molecular geometry.
Another misconception is that molecular geometry is a static concept. In reality, molecular geometry can be dynamic, influenced by factors such as temperature, pressure, and the presence of other molecules. For instance, water, as mentioned earlier, can exist in different phases, each with its unique molecular geometry.
Expert Perspective: Insights from a Leading Chemist
“The study of molecular geometry is a testament to the beauty and complexity of nature. It is a field that bridges the gap between the microscopic world of atoms and the macroscopic world we observe. By understanding the geometry of molecules, we gain insights into the fundamental principles that govern the behavior of matter. This knowledge has far-reaching implications, from the development of life-saving drugs to the design of sustainable materials. It is a field that continues to captivate and inspire scientists, offering endless possibilities for exploration and innovation.”
- Dr. Emily Anderson, Professor of Chemistry at Harvard University
Conclusion: A Never-Ending Quest for Understanding
In the realm of molecular geometry, every molecule tells a unique story, revealing the intricate interplay of atoms and their electrons. From the historical pioneers who laid the foundation to the modern scientists pushing the boundaries of knowledge, this field continues to evolve and inspire. As we delve deeper into the mysteries of molecular geometry, we unlock new possibilities, from enhancing our understanding of the natural world to creating innovative solutions for a better future. The journey is ongoing, and the mysteries yet to be unraveled are as exciting as they are vast.
The study of molecular geometry is a dynamic and ever-evolving field, offering profound insights into the structure and behavior of matter. From its historical beginnings to the cutting-edge technologies of today, this discipline continues to shape our understanding of the world, impacting fields as diverse as medicine, materials science, and environmental studies.
How does molecular geometry influence chemical reactions?
+Molecular geometry plays a crucial role in determining the outcome of chemical reactions. The spatial arrangement of atoms within a molecule can affect its reactivity, stability, and interaction with other molecules. For instance, the geometry of a molecule can determine the type and strength of chemical bonds formed during a reaction, influencing the reaction’s speed and selectivity.
Can molecular geometry be altered, and if so, how?
+Yes, molecular geometry can indeed be altered. This is often achieved through chemical reactions that involve bond breaking and formation. By manipulating the conditions, such as temperature, pressure, or the presence of catalysts, scientists can induce changes in molecular geometry. Additionally, advanced techniques like molecular modeling and computational chemistry allow for the prediction and simulation of different molecular geometries.
What are some real-world applications of molecular geometry outside of chemistry?
+The principles of molecular geometry extend far beyond the realm of chemistry. In biology, for instance, molecular geometry is crucial for understanding the structure and function of biomolecules like proteins and DNA. In materials science, it plays a vital role in designing materials with specific properties, such as the creation of advanced polymers and nanomaterials. Even in fields like environmental science, molecular geometry is used to study the behavior of pollutants and their interactions with natural systems.
How does molecular geometry contribute to the development of new technologies?
+Molecular geometry is a fundamental concept in the development of new technologies. For example, in the field of electronics, the geometry of semiconductor materials determines their electrical properties, influencing the design of microchips and other electronic components. In renewable energy, the molecular geometry of materials used in solar cells and batteries plays a crucial role in their efficiency and performance. Understanding molecular geometry allows scientists and engineers to tailor materials for specific technological applications.
What are some challenges faced in studying molecular geometry, and how are they being addressed?
+One of the primary challenges in studying molecular geometry is the complexity of certain molecular systems. Some molecules, especially those with multiple heavy atoms, can have highly complex geometries that are challenging to predict and visualize. To address this, researchers are developing advanced computational methods and high-performance computing techniques to simulate and model these complex systems. Additionally, experimental techniques like X-ray diffraction and cryo-electron microscopy provide detailed structural information, helping to validate theoretical predictions.



