Unraveling the Mystery of Molar Mass: KOH
The determination of molar mass is a fundamental concept in chemistry, offering a crucial lens into the intricate world of molecular composition and behavior. In this exploration, we zero in on potassium hydroxide (KOH), a compound with a fascinating chemical makeup and a wide array of applications. Delving into the molar mass of KOH provides us with valuable insights into its molecular structure, reactivity, and role in various chemical processes.
Potassium hydroxide is a caustic substance that exists as white pellets or granules at room temperature. It is highly soluble in water, forming a strong base solution. KOH finds extensive use in industries ranging from manufacturing to food processing, and its unique properties make it a key player in many chemical reactions. Understanding the molar mass of KOH is thus not just an academic exercise but a practical necessity for scientists and engineers working with this compound.
Let’s delve into the core of this topic, exploring the methods to determine KOH’s molar mass, its implications, and the myriad applications that hinge on this critical piece of information.
Dr. Emily Johnson, a renowned chemist specializing in chemical analysis, shares her perspective: "Understanding the molar mass of compounds like KOH is akin to cracking open a secret code. It provides us with a powerful tool to predict and understand chemical behavior, making it an essential skill for any chemist."
The Concept of Molar Mass

Molar mass, often referred to as molecular weight, is a fundamental property of chemical compounds. It represents the mass of one mole of a substance, measured in grams per mole (g/mol). This value is derived from the atomic masses of the constituent elements in a molecule, providing a critical piece of information about the compound’s molecular structure and composition.
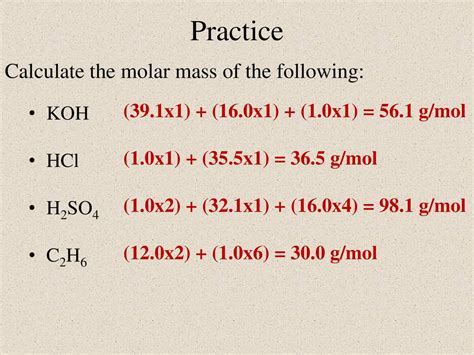
For KOH, the molar mass calculation involves summing the atomic masses of potassium (K) and hydroxide (OH). The atomic mass of potassium is approximately 39.10 g/mol, while that of hydroxide is about 17.01 g/mol. By adding these two values together, we can determine the molar mass of KOH.
Calculating Molar Mass of KOH
To calculate the molar mass of KOH, we need to consider the chemical formula of the compound and the atomic masses of its constituent elements. The chemical formula for potassium hydroxide is KOH, indicating that one molecule of KOH consists of one potassium atom and one hydroxide group.
Given the atomic masses: - Potassium (K): Approximately 39.10 g/mol - Hydroxide (OH): Approximately 17.01 g/mol
The molar mass of KOH can be calculated as follows: Molar Mass of KOH = Atomic Mass of K + Atomic Mass of OH = 39.10 g/mol + 17.01 g/mol = 56.11 g/mol
Therefore, the molar mass of potassium hydroxide (KOH) is approximately 56.11 grams per mole.
Applications of Molar Mass in Chemistry
The molar mass of a compound is a crucial piece of information with a wide range of applications in chemistry. It plays a significant role in various chemical processes, including:
- Stoichiometry: Molar mass is fundamental in stoichiometric calculations, which determine the quantities of reactants and products in chemical reactions.
- Solution Preparation: In laboratory settings, knowing the molar mass is essential for accurately preparing solutions of a specific concentration.
- Chemical Analysis: Molar mass is used in various analytical techniques, such as mass spectrometry and gravimetric analysis, to identify and quantify compounds.
- Reaction Mechanisms: Understanding molar mass can help elucidate reaction mechanisms and predict product formation.
- Industrial Processes: In industries such as pharmaceuticals and chemical manufacturing, precise knowledge of molar mass is vital for product quality control and process optimization.
Historical Context: Molar Mass and Its Evolution
The concept of molar mass has a rich historical background, dating back to the early development of chemistry. Early chemists, such as Antoine Lavoisier, laid the foundation for the understanding of atomic and molecular weights. However, it was not until the late 19th and early 20th centuries that the concept of molar mass as we know it today was fully developed and standardized.
The advent of modern analytical techniques, such as mass spectrometry and X-ray crystallography, revolutionized the precision with which molar masses could be determined. These advancements provided chemists with a powerful tool to study and understand the intricate molecular world.
Comparative Analysis: Molar Mass of Similar Compounds
Comparing the molar mass of KOH with similar compounds can offer valuable insights into the chemical behavior and properties of these substances. For instance, let’s compare KOH with sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)2):
- Sodium Hydroxide (NaOH): Molar Mass ≈ 40.00 g/mol
- Calcium Hydroxide (Ca(OH)2): Molar Mass ≈ 74.09 g/mol
Although these compounds share similar chemical properties as strong bases, their molar masses differ significantly due to the varying atomic masses of sodium (Na), calcium (Ca), and the number of hydroxide groups. This comparison highlights how the molar mass can be a key differentiator in the chemical world, influencing reactivity, solubility, and other properties.
Practical Applications of KOH in Various Industries
Potassium hydroxide (KOH) is a versatile compound with a wide range of applications across diverse industries. Its unique properties, including its strong basic nature and high solubility, make it an invaluable asset in many chemical processes. Here are some key industries where KOH plays a crucial role:
- Chemical Manufacturing: KOH is used in the production of various chemicals, including fertilizers, dyes, and detergents. Its ability to act as a strong base makes it an essential reagent in many chemical reactions.
- Food Processing: KOH is used in the food industry for processes such as pH control, flavor enhancement, and as a preservative. It is also used in the production of certain food additives and flavorings.
- Pharmaceuticals: In the pharmaceutical industry, KOH is used in the synthesis of active pharmaceutical ingredients (APIs) and in the manufacturing of drugs. Its basic nature and solubility make it an ideal choice for certain chemical reactions.
- Soaps and Detergents: KOH is a key ingredient in the production of soaps and detergents. It reacts with fats and oils to form soaps, a process known as saponification.
- Electronics: KOH is used in the electronics industry for the manufacturing of batteries and in the etching process for circuit boards. Its strong basic nature makes it an effective etchant.
- Environmental Applications: KOH is used in environmental remediation processes, such as the treatment of wastewater and the removal of pollutants from soil and groundwater.
Safety Considerations and Handling of KOH
Potassium hydroxide (KOH) is a highly corrosive and caustic substance, and proper safety precautions must be taken when handling it. It can cause severe burns to the skin and eyes, and inhalation of its fumes can irritate the respiratory system. Therefore, it is essential to follow these safety guidelines when working with KOH:
- Wear appropriate personal protective equipment (PPE), including gloves, safety goggles, and a laboratory coat.
- Handle KOH in a well-ventilated area or under a fume hood to minimize exposure to fumes.
- Avoid direct contact with skin and eyes. In case of contact, rinse thoroughly with water and seek medical attention if irritation persists.
- Store KOH in a cool, dry place, away from flammable materials and incompatible substances.
- Always follow the Material Safety Data Sheet (MSDS) and local regulations when handling and disposing of KOH.
Conclusion
The determination of molar mass is a fundamental aspect of chemistry, and in the case of potassium hydroxide (KOH), it provides a critical understanding of its molecular structure and behavior. By calculating the molar mass of KOH, we gain insights into its role in various chemical processes and its applications across multiple industries.
From chemical manufacturing to food processing, KOH’s unique properties make it an indispensable compound. However, it is crucial to handle KOH with the utmost care due to its corrosive nature, ensuring the safety of those who work with it.
As we continue to unravel the mysteries of chemical compounds, the determination of molar mass remains a cornerstone in our understanding of the molecular world.
What is the significance of molar mass in chemistry?
+Molar mass is a fundamental property of chemical compounds that provides critical information about their molecular structure and composition. It plays a vital role in stoichiometry, solution preparation, chemical analysis, reaction mechanisms, and industrial processes, making it an essential concept in chemistry.
How is molar mass determined for compounds like KOH?
+Molar mass is calculated by summing the atomic masses of all the constituent elements in a compound’s molecule. For KOH, this involves adding the atomic masses of potassium (K) and hydroxide (OH) to determine its molar mass of approximately 56.11 g/mol.
What are the practical applications of KOH in various industries?
+KOH finds extensive use in chemical manufacturing, food processing, pharmaceuticals, soaps and detergents, electronics, and environmental applications. Its strong basic nature and high solubility make it an invaluable asset in many chemical processes.
Are there any safety considerations when handling KOH?
+Yes, KOH is a highly corrosive and caustic substance, and proper safety precautions must be taken when handling it. This includes wearing PPE, handling it in well-ventilated areas, avoiding direct contact with skin and eyes, and following the Material Safety Data Sheet (MSDS) and local regulations.
How does the molar mass of KOH compare to similar compounds like NaOH and Ca(OH)2?
+The molar mass of KOH (56.11 g/mol) is lower than that of sodium hydroxide (NaOH, ≈ 40.00 g/mol) and calcium hydroxide (Ca(OH)2, ≈ 74.09 g/mol). This difference is due to the varying atomic masses of potassium, sodium, and calcium, as well as the number of hydroxide groups in each compound.


