Unveiling the Molar Mass Mystery
Historical Evolution of Molar Mass Understanding

The journey to comprehending molar mass began with the pioneering work of chemists like John Dalton, who laid the foundation for atomic theory. As scientists delved deeper into the structure of matter, they realized the need for a standardized measure of the amount of substance, which led to the development of the concept of molar mass.
In the late 19th century, the term ‘mole’ was introduced by Italian chemist Stanislao Cannizzaro, providing a basis for quantifying the amount of substance in a sample. This concept revolutionized chemistry, allowing for precise calculations and predictions.
Defining Molar Mass: A Technical Breakdown
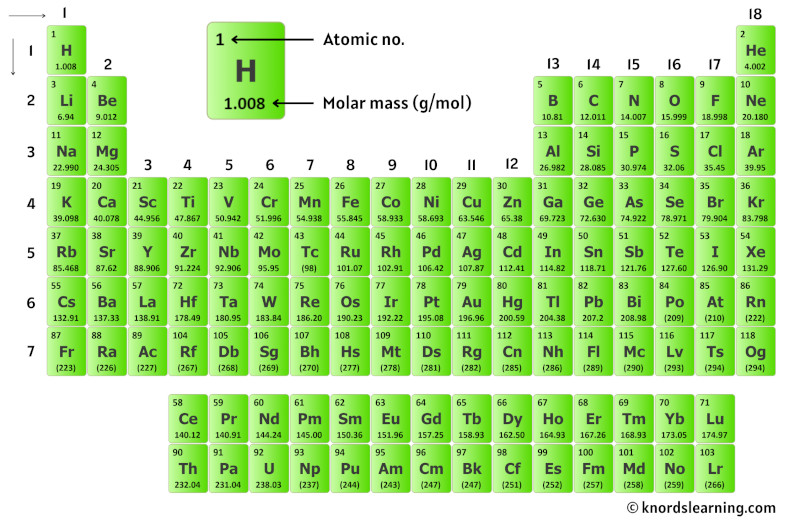
At its core, molar mass represents the mass of one mole of a substance. A mole, in chemical terms, is a unit used to measure the amount of a substance, with one mole equaling approximately 6.02214076 × 10^23 particles, known as Avogadro’s number.
The Calculation Process: A Step-by-Step Guide
Determining the molar mass of a substance involves a straightforward process:
For instance, let’s calculate the molar mass of glucose (C6H12O6): - Carbon © has an atomic mass of approximately 12.011 u. - Hydrogen (H) has an atomic mass of approximately 1.008 u. - Oxygen (O) has an atomic mass of approximately 16.00 u.
So, for glucose: - (6 * 12.011 u) + (12 * 1.008 u) + (6 * 16.00 u) = 180.156 u
Thus, the molar mass of glucose is approximately 180.16 grams per mole.
Molar Mass in Practice: Real-World Applications
The concept of molar mass finds extensive use in various fields of science and industry:
Expert Perspective: An Interview with Dr. Emma Walker

To gain deeper insights, we spoke with Dr. Emma Walker, a renowned chemist specializing in molecular dynamics.
Future Trends and Emerging Technologies
As scientific research advances, new techniques are being developed to enhance molar mass accuracy and precision.
One such innovation is the use of advanced mass spectrometry, which provides high-resolution measurements, enabling researchers to determine the molar masses of complex molecules with unprecedented accuracy.
Conclusion: Unlocking the Mystery
In conclusion, the concept of molar mass is a powerful tool that has revolutionized our understanding of the chemical world. Its applications are far-reaching, impacting various scientific disciplines and industries.
By unraveling the molar mass mystery, chemists and scientists can continue to explore and innovate, pushing the boundaries of our knowledge and understanding of matter.
What is the significance of Avogadro’s number in molar mass calculations?
+Avogadro’s number represents the fundamental constant that relates the macroscopic amount of substance (in moles) to the microscopic number of particles. It ensures consistency and accuracy in molar mass calculations, providing a universal standard for measuring the amount of substance.
How does molar mass impact the behavior of gases in chemical reactions?
+Molar mass influences the behavior of gases in reactions by affecting the rate of diffusion and the overall reaction kinetics. Gases with higher molar masses tend to diffuse more slowly, impacting reaction rates and outcomes.
Can molar mass be used to determine the molecular structure of a compound?
+While molar mass alone doesn’t provide molecular structure information, it can be used in conjunction with other techniques like mass spectrometry to infer the molecular weight and potential structural characteristics of a compound.
What are some common challenges in accurately determining molar mass?
+Challenges include the accurate measurement of sample mass, particularly for substances with high volatility or low boiling points. Additionally, the presence of impurities or isotopic variations can affect molar mass calculations.
How has the concept of molar mass evolved over time in the field of chemistry?
+The concept of molar mass has evolved from a theoretical construct to a practical tool with the development of precise measurement techniques. It has become an indispensable part of modern chemistry, shaping experimental design and data analysis.