How to Calculate Calcium Carbonate's Molar Mass
Calcium carbonate (CaCO3) is a common compound found in nature, and understanding its molar mass is essential for various scientific and industrial applications. Let’s dive into the process of calculating its molar mass and explore its significance.
To determine the molar mass of calcium carbonate, we need to consider the atomic masses of its constituent elements: calcium (Ca), carbon ©, and oxygen (O). These atomic masses can be found on the periodic table, which provides the fundamental building blocks for our calculation.
Here’s a step-by-step guide to calculating the molar mass of calcium carbonate:
Identify the Elements: Start by recognizing that calcium carbonate is composed of calcium, carbon, and oxygen atoms.
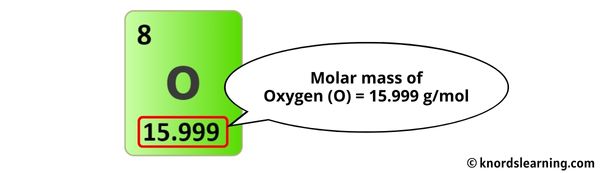
Find Atomic Masses: Refer to the periodic table to locate the atomic masses of each element. For calcium, carbon, and oxygen, the atomic masses are approximately 40, 12, and 16, respectively.
Calculate the Molar Mass: Multiply the atomic mass of each element by the number of atoms present in the compound and then sum up these values. In the case of calcium carbonate:
- Calcium (Ca): 40 atomic mass units (amu) x 1 atom = 40 amu
- Carbon ©: 12 amu x 1 atom = 12 amu
- Oxygen (O): 16 amu x 3 atoms = 48 amu Now, add these values together: 40 amu + 12 amu + 48 amu = 100 amu.
Express in Grams: To convert the molar mass from atomic mass units to grams, we use the definition of a mole. One mole of any substance has a mass equal to its molar mass in grams. Therefore, the molar mass of calcium carbonate is approximately 100 grams per mole (g/mol).
In simpler terms, if you have one mole of calcium carbonate, it will weigh approximately 100 grams. This molar mass is a fundamental property of the compound and plays a crucial role in various scientific calculations and experiments.
Let’s delve into a practical scenario to illustrate the significance of calcium carbonate’s molar mass:
Imagine you are a chemist working in a laboratory, and you need to prepare a specific concentration of a calcium carbonate solution for an experiment. By knowing the molar mass, you can accurately calculate the amount of calcium carbonate required to achieve the desired concentration. This precision is essential for ensuring the reliability and reproducibility of your experimental results.
Furthermore, the molar mass of calcium carbonate is vital in various industries. For instance, in the pharmaceutical industry, precise control over the dosage of calcium carbonate supplements is necessary for patient safety and efficacy. The molar mass calculation ensures that the correct amount of the compound is administered.
In summary, calculating the molar mass of calcium carbonate involves identifying its constituent elements, finding their atomic masses, and performing a simple multiplication and addition operation. The resulting molar mass, approximately 100 g/mol, is a fundamental property with practical applications in scientific research, experimentation, and industrial processes. Understanding and applying this concept contributes to the accuracy and reliability of numerous chemical and biological endeavors.



