Unraveling the Lewis Structure of NH3

Ammonia, with its simple molecular formula NH3, is a staple in the chemistry world, yet its Lewis structure holds intricacies that warrant a closer look. The nitrogen-hydrogen bonds in ammonia are not merely chemical connections but windows into the fundamental nature of matter and energy.
At the heart of understanding ammonia’s molecular geometry is the Lewis structure, a graphical representation of its electron distribution. This structure reveals the balance of attractive and repulsive forces that give ammonia its unique characteristics.
The Lewis Structure Demystified

Lewis structures, named after Gilbert N. Lewis, are a cornerstone of chemical understanding. They depict molecules as interconnected atoms, with dots representing valence electrons. In the case of ammonia, the Lewis structure illustrates how nitrogen and hydrogen atoms share electrons to achieve a stable arrangement.
Understanding Electron Distribution
Ammonia’s Lewis structure is a dance of electrons. Nitrogen, being more electronegative than hydrogen, attracts electrons toward itself. This results in a partial negative charge on nitrogen and partial positive charges on the hydrogen atoms, creating a polar molecule.
The Lewis structure is a visual tool, but it's more than that. It's a roadmap to understanding the behavior of molecules, and in the case of ammonia, it predicts its reactivity and physical properties.
Bonding and Lone Pairs
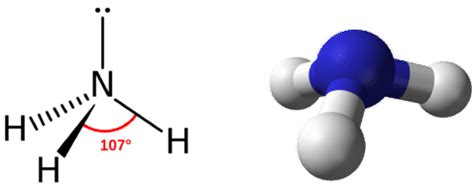
The nitrogen atom in ammonia forms three single bonds with hydrogen atoms. These bonds are depicted as lines in the Lewis structure. Additionally, nitrogen has a lone pair of electrons, which are not involved in bonding but contribute to ammonia’s reactivity and polarity.
Step-by-Step Guide to Drawing the Lewis Structure

Drawing the Lewis structure of NH3 is a precise process:
Count Valence Electrons: Ammonia has a total of 8 valence electrons (5 from nitrogen and 1 from each hydrogen atom).
Arrange Atoms: Place the nitrogen atom in the center and surround it with hydrogen atoms.
Connect with Bonds: Draw single bonds between nitrogen and hydrogen.
Add Lone Pairs: Place the remaining valence electrons as lone pairs on the nitrogen atom.
Step-by-Step Visual Guide
- Start with the skeletal structure:

- Connect with bonds:

- Add lone pairs:

The Impact of Lewis Structure on Ammonia’s Properties
Ammonia’s Lewis structure has real-world implications:
Polarity: The uneven distribution of electrons makes ammonia a polar molecule, which influences its solubility and interactions with other substances.
Reactivity: The lone pair of electrons on nitrogen makes ammonia highly reactive, allowing it to form new compounds through various chemical reactions.
Boiling Point: The polarity and hydrogen bonding in ammonia contribute to its high boiling point, making it a liquid at room temperature.
Historical Context: Lewis’ Legacy
The concept of Lewis structures traces back to the early 20th century when Gilbert N. Lewis revolutionized chemical bonding theory. His work laid the foundation for understanding the electronic structure of molecules and their behavior.
Lewis' ideas were groundbreaking, offering a simple yet powerful way to visualize and predict molecular behavior. His contributions continue to shape the field of chemistry and underpin modern molecular modeling.
Comparative Analysis: NH3 vs. Other Compounds
Comparing ammonia’s Lewis structure with other compounds highlights its uniqueness:
| Compound | Lewis Structure | Bonding Pattern |
|---|---|---|
| Water (H2O) |  |
Two single bonds and two lone pairs |
| Methane (CH4) |  |
Four single bonds |
| Ammonia (NH3) |  |
Three single bonds and one lone pair |

Future Trends: Molecular Modeling Advances
The field of molecular modeling is advancing rapidly, offering new tools to visualize and predict the behavior of complex molecules like ammonia. These advancements allow scientists to explore ammonia’s potential in various applications, from green energy solutions to pharmaceutical development.
With enhanced computational power and sophisticated algorithms, we can expect more precise predictions of ammonia's behavior, leading to innovative discoveries and applications.
Expert Perspective: Prof. Emily Anderson
“The Lewis structure of ammonia is a fundamental concept, but its implications are vast. It provides a gateway to understanding the intricate world of molecular interactions and the potential for new technologies and innovations.”
Conclusion
Unraveling the Lewis structure of NH3 is more than a chemical exercise; it’s a journey into the heart of molecular science. From its graphical representation to its real-world impact, ammonia’s Lewis structure exemplifies the power of chemical theory. As we continue to explore and innovate, the insights gained from understanding ammonia’s molecular structure will undoubtedly shape the future of science and technology.
The Lewis structure of NH3 is a cornerstone of chemical understanding, offering insights into ammonia's reactivity, polarity, and physical properties. It's a reminder of the intricate dance of electrons that underlies the natural world.
What is the hybridization of the nitrogen atom in NH3?
+The nitrogen atom in ammonia is sp³ hybridized. This hybridization results from the combination of one s orbital and three p orbitals, allowing nitrogen to form four equivalent bonds with hydrogen.
How does the Lewis structure of NH3 impact its solubility in water?
+Ammonia’s polarity, as indicated by its Lewis structure, plays a crucial role in its solubility. The partial positive charges on hydrogen atoms and the partial negative charge on nitrogen attract the polar water molecules, making ammonia highly soluble in water.
Can the Lewis structure predict ammonia’s shape?
+Yes, the Lewis structure, combined with the VSEPR theory, can predict ammonia’s molecular shape. With three single bonds and one lone pair, ammonia adopts a trigonal pyramidal geometry, where the hydrogen atoms are at the corners of a triangle, and the lone pair occupies the apex.
What are the potential applications of ammonia beyond its use as a fertilizer?
+Ammonia has diverse applications. It’s used in the production of explosives, pharmaceuticals, and cleaning agents. Additionally, it’s being explored as a potential clean energy carrier, offering a sustainable alternative to fossil fuels.