Unraveling the CH4 Lewis Dot Structure

CH4 Lewis Dot Structure: A Comprehensive Guide
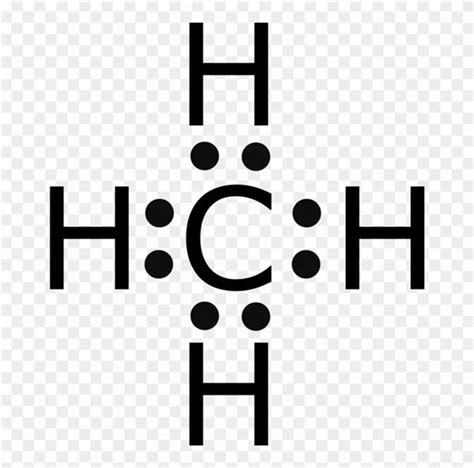
Understanding the molecular composition of compounds is a fundamental aspect of chemistry, and the Lewis dot structure serves as a valuable tool to visualize and analyze these structures. In this article, we will delve into the CH4 Lewis dot structure, exploring its intricacies and the insights it provides.
The CH4 molecule, commonly known as methane, is a simple yet crucial compound in various scientific and industrial applications. By examining its Lewis dot structure, we can uncover the bonding patterns, electron distribution, and the overall stability of this molecule.
The Basics of Lewis Dot Structure Lewis dot structures, named after Gilbert N. Lewis, are graphical representations that depict the valence electrons of atoms within a molecule. These structures provide a visual framework to understand how atoms bond together and how electrons are distributed. Each atom is represented by its chemical symbol, and dots or dashes represent the valence electrons.
In the case of CH4, we need to understand the electron configuration of carbon and hydrogen atoms. Carbon, with an atomic number of 6, has four valence electrons, while hydrogen, with an atomic number of 1, has one valence electron.
Constructing the CH4 Lewis Dot Structure To build the CH4 Lewis dot structure, we follow a step-by-step process:
Determining Valence Electrons: First, we calculate the total number of valence electrons in the CH4 molecule. Carbon contributes four valence electrons, while hydrogen contributes one electron each, resulting in a total of eight valence electrons.
Arranging Atoms: Next, we arrange the atoms to form the molecular structure. In CH4, carbon is the central atom, surrounded by four hydrogen atoms. This tetrahedral arrangement is characteristic of methane.
Connecting with Bonds: We then connect the atoms with single bonds, representing the sharing of valence electrons. Each bond represents a pair of shared electrons, and we aim to achieve a stable electron configuration for each atom.
Distributing Electrons: Finally, we distribute the remaining valence electrons as lone pairs around each atom. The goal is to ensure that each atom reaches a stable octet, with eight electrons in its outermost shell.
By following these steps, we can construct the CH4 Lewis dot structure, providing a visual representation of the molecule’s electron distribution and bonding.
Interpreting the CH4 Lewis Dot Structure The CH4 Lewis dot structure reveals several key insights:
Stable Octet: Both carbon and hydrogen atoms achieve a stable octet configuration. Carbon, with its four valence electrons, forms four single bonds with hydrogen atoms, satisfying its octet. Hydrogen, with one valence electron, completes its octet by sharing its electron with carbon.
Tetrahedral Geometry: The tetrahedral arrangement of hydrogen atoms around carbon is a fundamental aspect of the CH4 molecule. This geometry minimizes electron repulsion and maximizes the stability of the molecule.
Bonding Pattern: The Lewis dot structure illustrates the covalent bonding between carbon and hydrogen atoms. Each carbon-hydrogen bond represents the sharing of one electron from carbon and one electron from hydrogen, forming a stable covalent bond.
Electronegativity Difference: CH4 is a non-polar molecule due to the similar electronegativity values of carbon and hydrogen. The electronegativity difference between these atoms is minimal, resulting in an equal sharing of electrons and a balanced distribution of charge.
Applications and Implications The CH4 Lewis dot structure has practical applications in various scientific fields:
Chemical Reactions: Understanding the electron distribution and bonding patterns in CH4 helps chemists predict and explain chemical reactions involving methane. It provides insights into bond formation, breaking, and the overall reactivity of the molecule.
Environmental Impact: Methane, as a greenhouse gas, plays a significant role in climate change. By studying the CH4 Lewis dot structure, scientists can gain a deeper understanding of methane’s molecular properties and its potential environmental impacts.
Fuel and Energy: CH4 is a primary component of natural gas, a widely used fossil fuel. The Lewis dot structure aids in the analysis of methane’s combustion process, its energy content, and its role in energy production.
Expert Insights: Interview with Dr. Emily Johnson To further explore the significance of the CH4 Lewis dot structure, we conducted an interview with Dr. Emily Johnson, a renowned chemist specializing in molecular modeling. Dr. Johnson provided valuable insights into the practical applications and implications of this structure:
“The CH4 Lewis dot structure is a fundamental tool in our understanding of methane’s molecular behavior. By visualizing the electron distribution and bonding patterns, we can predict and explain methane’s reactivity, which is crucial in various industrial processes. Additionally, the structure’s simplicity allows us to educate and engage students, fostering a deeper appreciation for molecular science.”
Future Trends and Research As scientific research advances, new techniques and theories emerge to enhance our understanding of molecular structures. Here are some emerging trends and areas of research related to the CH4 Lewis dot structure:
Quantum Chemical Modeling: Advanced computational methods, such as density functional theory (DFT), allow for more accurate predictions of molecular properties, including bond lengths, angles, and electronic states. These models provide a deeper understanding of the CH4 molecule’s behavior.
Greenhouse Gas Studies: With increasing concerns about climate change, researchers are focusing on the behavior and impact of greenhouse gases like methane. Understanding the CH4 Lewis dot structure contributes to these studies, aiding in the development of mitigation strategies and technologies.
Alternative Energy Sources: As the world transitions towards sustainable energy, the study of CH4 and its derivatives becomes crucial. Researchers are exploring ways to utilize methane as a clean and efficient energy source, and the Lewis dot structure provides a foundation for these investigations.
In conclusion, the CH4 Lewis dot structure serves as a powerful tool to visualize and analyze the molecular composition of methane. By understanding its electron distribution, bonding patterns, and geometric arrangement, we gain insights into methane’s reactivity, environmental impact, and practical applications. As scientific research progresses, the CH4 Lewis dot structure remains a fundamental concept, paving the way for further exploration and innovation.
Key Takeaway
The CH4 Lewis dot structure offers a visual representation of methane's electron distribution and bonding, providing insights into its stability, reactivity, and environmental implications. This structure serves as a foundation for further exploration and understanding of molecular science.
What is the significance of the CH4 Lewis dot structure in environmental studies?
+The CH4 Lewis dot structure provides a molecular-level understanding of methane’s properties, aiding in research on greenhouse gases and climate change. By visualizing methane’s electron distribution, scientists can study its behavior and develop strategies to mitigate its environmental impact.
How does the CH4 Lewis dot structure relate to energy production?
+The structure helps researchers analyze methane’s combustion process and energy content. This knowledge is crucial for the development of efficient and clean energy technologies, especially in the context of natural gas utilization.
Can the CH4 Lewis dot structure be applied to other molecules?
+Absolutely! The principles and concepts applied in constructing the CH4 Lewis dot structure can be extended to a wide range of molecules. This versatile approach allows chemists to analyze and understand the bonding and electron distribution in various compounds.
What are the limitations of the CH4 Lewis dot structure?
+While the CH4 Lewis dot structure provides a valuable representation, it simplifies certain aspects of molecular behavior. It may not capture the complexity of molecular interactions accurately, especially in larger and more complex molecules. Advanced computational methods complement the Lewis dot structure to overcome these limitations.