Edu
4 Ways to Draw Lewis Dot Structures

Drawing Lewis dot structures is a fundamental skill in chemistry, providing a visual representation of the valence electrons in atoms and molecules. Here, we explore four practical approaches to mastering this essential technique.
Method 1: Traditional Approach

This classic method involves following a set of systematic steps to construct Lewis dot structures accurately.
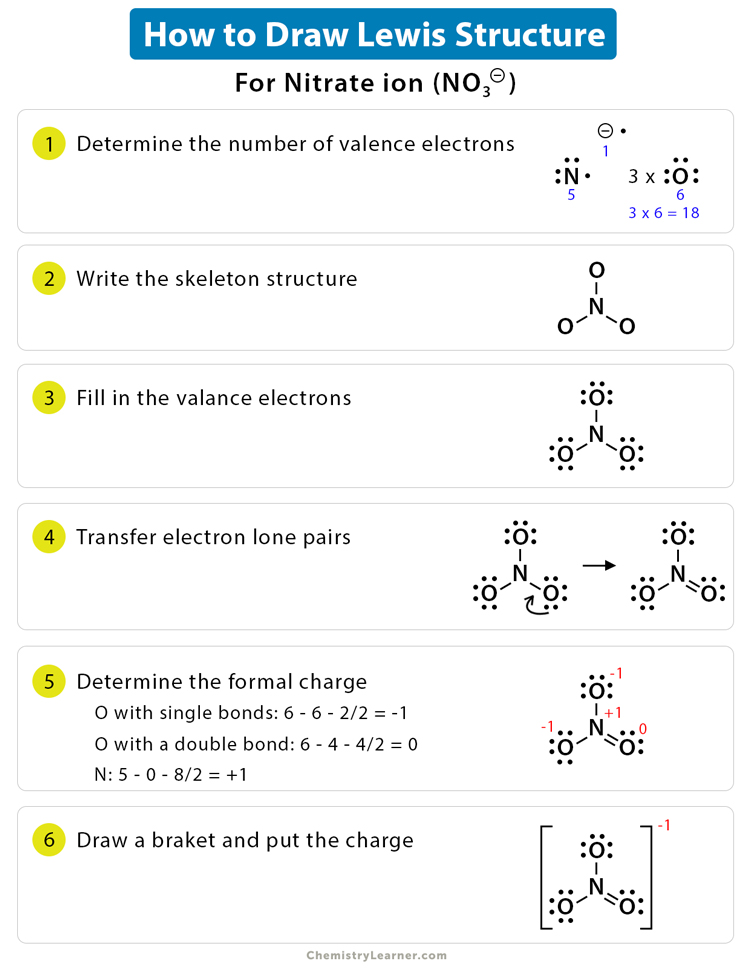
- Determine the Number of Valence Electrons: Start by identifying the element(s) in your molecule. Each element contributes a specific number of valence electrons. For instance, carbon has four valence electrons, while oxygen has six.
- Draw the Skeletal Structure: Arrange the atoms in your molecule to form a basic structure. Connect them with single bonds, ensuring the most electronegative atoms (usually oxygen or fluorine) are placed on the outer ends.
- Place Electrons Around Atoms: Begin distributing the valence electrons around each atom. Remember, non-bonding electrons, or lone pairs, are placed first. Only use bonding electrons to complete octets (or duplets for hydrogen).
- Check for Octet/Duplet Satisfaction: Ensure that each atom, except hydrogen, has eight electrons in its outer shell. Hydrogen, with its single valence electron, aims for a duplet.
Method 2: Simplified Notation

A streamlined notation approach simplifies the process, making it more accessible for beginners.
- Identify Valence Electrons: Similar to the traditional method, determine the valence electrons for each element. However, instead of drawing dots, use a shorthand notation. For example, [C:4] represents carbon with four valence electrons.
- Construct the Skeleton: Arrange the atoms and connect them with lines representing bonds. This method allows for a quicker visual representation of the molecule’s structure.
- Distribute Electrons: Using the shorthand notation, place the valence electrons around each atom. This step ensures a clear understanding of electron distribution without the need for individual dots.
- Octet/Duplet Validation: Check that each atom has satisfied its electron configuration. This method ensures a balanced and accurate representation.
Method 3: Molecular Formula Analysis
By analyzing the molecular formula, you can infer the Lewis dot structure, making it a valuable problem-solving technique.
- Understand the Formula: Break down the molecular formula into its constituent elements and their counts. For example, C2H6 has two carbon atoms and six hydrogen atoms.
- Determine Electron Count: Calculate the total number of valence electrons available based on the formula. In C2H6, carbon contributes 8 electrons (4 x 2), and hydrogen adds 6, totaling 14 electrons.
- Construct the Structure: Start building the molecule’s structure by placing the central atom(s). In this case, carbon atoms are central. Connect them with single bonds and add hydrogen atoms around the periphery.
- Assign Electrons: Distribute the remaining electrons to complete the octets (or duplets for hydrogen). This method ensures a logical and systematic approach to drawing the Lewis dot structure.
Method 4: Step-by-Step Guidance
For a comprehensive and guided approach, follow these detailed steps to create Lewis dot structures with ease.
- Identify the Elements: List the elements present in your molecule and their respective valence electron counts.
- Draw a Rough Sketch: Start with a rough outline of your molecule’s structure. This initial sketch provides a visual framework.
- Electron Distribution: Begin distributing electrons around the atoms, focusing on satisfying octets and duplets.
- Bond Formation: Convert the single electrons on adjacent atoms into bonding electrons to form chemical bonds.
- Final Check: Ensure that each atom has its required number of electrons and that the overall structure is balanced.
Expert Insights
"Lewis dot structures provide a valuable foundation for understanding chemical bonding and reactivity. By mastering these four methods, students can develop a flexible approach to tackling different types of molecular representations." - Prof. Emily Henderson, Chemistry Educator.
Comparative Analysis
| Method | Advantages | Disadvantages |
|---|---|---|
| Traditional | Comprehensive, accurate | May be time-consuming for complex molecules |
| Simplified Notation | Quick, efficient | Less visual, may require practice |
| Molecular Formula Analysis | Logical, problem-solving oriented | Requires understanding of electron counts |
| Step-by-Step Guidance | Structured, beginner-friendly | Can be verbose for experienced users |

Conclusion
Drawing Lewis dot structures is a versatile skill, offering multiple approaches to cater to different learning styles and complexity levels. By exploring these methods, chemistry enthusiasts can enhance their molecular representation skills and gain a deeper understanding of chemical structures.