Unleash Your Creativity with Lewis Diagram Tools
Unleashing Creative Potential: A Journey into the World of Lewis Diagram Tools

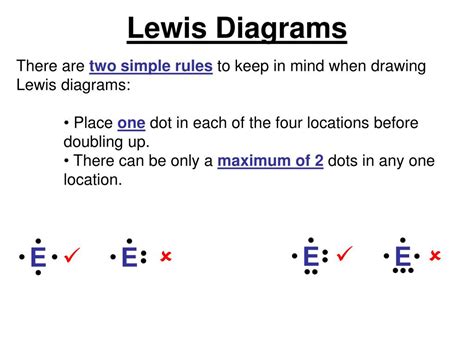
Lewis diagrams, also known as electron dot diagrams or Lewis dot structures, are powerful visual tools that have revolutionized the way we understand and represent chemical bonding and molecular geometry. These diagrams provide a simple yet elegant method to depict the distribution of electrons in atoms, molecules, and ions, offering invaluable insights into the intricate world of chemistry.
In this article, we embark on a comprehensive exploration of Lewis diagram tools, delving into their history, significance, and practical applications. We will discover how these diagrams have become an indispensable resource for chemists, students, and anyone fascinated by the building blocks of our universe.
The Historical Evolution of Lewis Diagrams
The concept of Lewis diagrams can be traced back to the early 20th century, when American chemist Gilbert N. Lewis proposed a new theory of chemical bonding. In a groundbreaking paper published in 1916, titled “The Atom and the Molecule,” Lewis introduced the idea that chemical bonds are formed by the sharing or transfer of electrons between atoms.
Lewis’s theory was a significant departure from previous understandings of chemical bonding, which often relied on more complex mathematical models. His simple yet powerful concept laid the foundation for a new way of visualizing and understanding the behavior of atoms and molecules.
The electron dot diagrams, which we now refer to as Lewis diagrams, were introduced as a visual representation of Lewis’s bonding theory. These diagrams provide a clear and intuitive way to illustrate the number of valence electrons in an atom, the distribution of these electrons, and the formation of chemical bonds.
Unlocking the Power of Lewis Diagrams
Lewis diagrams offer a wealth of benefits to those exploring the world of chemistry:
Simplified Representation: Lewis diagrams provide a straightforward and easily understandable representation of complex chemical concepts. By using simple dots to represent valence electrons, these diagrams offer a visual language that is accessible to both experts and beginners alike.
Bonding Insights: One of the primary advantages of Lewis diagrams is their ability to reveal the nature of chemical bonding. By depicting the sharing or transfer of electrons, these diagrams provide a clear picture of how atoms form bonds and interact with one another.
Molecular Geometry: Lewis diagrams are not just limited to representing individual atoms. They can also be used to depict the geometry of molecules, showing the arrangement of atoms within a molecule and the direction of chemical bonds. This information is crucial for understanding molecular behavior and properties.
Educational Tool: Lewis diagrams have become an essential teaching tool in chemistry education. They help students visualize and understand the fundamental concepts of chemical bonding, atomic structure, and molecular geometry.
Research and Analysis: For researchers and scientists, Lewis diagrams are invaluable for analyzing and predicting the behavior of chemical compounds. By studying the distribution of electrons and the resulting bond formations, researchers can make informed predictions about molecular stability, reactivity, and other chemical properties.
Practical Applications: Where Lewis Diagrams Come to Life
The applications of Lewis diagrams extend far beyond the theoretical realm. Here are some practical scenarios where these diagrams are used:
Chemical Synthesis: In the field of synthetic chemistry, Lewis diagrams are crucial for planning and understanding chemical reactions. By visualizing the electron distribution before and after a reaction, chemists can predict the products and by-products formed, ensuring more efficient and accurate synthesis processes.
Pharmaceutical Research: The pharmaceutical industry relies heavily on Lewis diagrams to understand the molecular structures of drugs and their interactions with biological systems. This knowledge is essential for drug design, development, and optimization.
Environmental Chemistry: Lewis diagrams play a vital role in environmental chemistry, where they are used to study the behavior of pollutants, toxins, and other chemical compounds in the environment. By understanding the molecular structure and bonding patterns, researchers can develop strategies to mitigate environmental hazards.
Materials Science: In the realm of materials science, Lewis diagrams are employed to understand the properties and behavior of various materials, from metals and alloys to polymers and ceramics. This knowledge is critical for developing new materials with specific properties and functionalities.
Forensic Chemistry: Forensic chemists use Lewis diagrams to analyze the molecular structures of substances found at crime scenes. By understanding the bonding patterns and electron distributions, they can identify unknown substances and gather crucial evidence for investigations.
The Future of Lewis Diagrams: Evolution and Expansion
While Lewis diagrams have already established themselves as an essential tool in chemistry, the future holds exciting possibilities for their continued development and expansion:
Digital Tools: With the advent of digital technologies, new software and online platforms are being developed to create and manipulate Lewis diagrams more efficiently. These tools offer enhanced visualization capabilities, allowing for more complex and interactive representations of molecular structures.
Integration with Artificial Intelligence: AI-powered systems are being integrated with Lewis diagram tools to automate the process of diagram generation and analysis. These systems can rapidly generate accurate Lewis diagrams for complex molecules, providing valuable insights for researchers and students.
Virtual Reality (VR) and Augmented Reality (AR): The integration of VR and AR technologies offers the potential to create immersive experiences where users can interact with and manipulate Lewis diagrams in three-dimensional spaces. This could revolutionize the way chemistry is taught and studied, providing a more engaging and interactive learning environment.
Expansion to Other Scientific Fields: While Lewis diagrams have traditionally been associated with chemistry, there is potential for their application in other scientific disciplines. For example, they could be used to represent the distribution of charge in electrical circuits or the flow of energy in ecological systems.
Conclusion: Embracing the Power of Lewis Diagrams
In conclusion, Lewis diagrams are more than just a tool for representing chemical structures. They are a powerful language that enables us to understand and communicate complex chemical concepts with simplicity and elegance. From their historical origins to their contemporary applications, Lewis diagrams have proven their worth as an indispensable resource for chemists and anyone interested in the fundamental building blocks of our world.
As we continue to explore and expand our understanding of the universe, Lewis diagrams will undoubtedly remain a cornerstone of chemical education and research, unlocking the secrets of the atomic and molecular realm.
Frequently Asked Questions

What is the primary purpose of Lewis diagrams in chemistry?
+Lewis diagrams serve as a visual representation of the distribution of electrons in atoms, molecules, and ions. They provide insights into chemical bonding, molecular geometry, and the behavior of chemical compounds, making them an essential tool for chemists and students.
How are Lewis diagrams used in chemical education?
+Lewis diagrams are widely used in chemistry education as a teaching tool. They help students visualize and understand the fundamental concepts of chemical bonding, atomic structure, and molecular geometry, making complex chemical concepts more accessible and engaging.
Can Lewis diagrams be applied to other scientific fields beyond chemistry?
+While Lewis diagrams have traditionally been associated with chemistry, there is growing interest in applying these diagrams to other scientific disciplines. For example, they could be used to represent the distribution of charge in electrical circuits or the flow of energy in ecological systems.
How have Lewis diagrams evolved with the advancement of technology?
+With the advent of digital technologies, new software and online platforms have been developed to create and manipulate Lewis diagrams more efficiently. These tools offer enhanced visualization capabilities and the potential for integration with AI and VR/AR technologies, revolutionizing the way Lewis diagrams are used and understood.