What You Need to Know About KCl Molar Mass

The molar mass of KCl, or potassium chloride, is a fundamental property that holds significance across various scientific and industrial applications. Understanding this concept is crucial for chemists, researchers, and professionals in fields such as medicine, agriculture, and environmental science.
Potassium chloride, a simple yet versatile compound, serves as a vital component in numerous processes, from fertilizer production to medical treatments. Its molar mass provides a key piece of information that influences its behavior, reactivity, and utility in different contexts.
So, what exactly is the molar mass of KCl, and why is it important to grasp this concept? Let’s delve into the details.
The Concept of Molar Mass
Molar mass, also known as molecular weight, is a fundamental property of substances that describes the mass of one mole of a particular substance. A mole is a unit of measurement in chemistry that represents a fixed number of particles, specifically 6.022 x 10^23 particles. This number, known as Avogadro’s number, is a constant that applies to all substances.
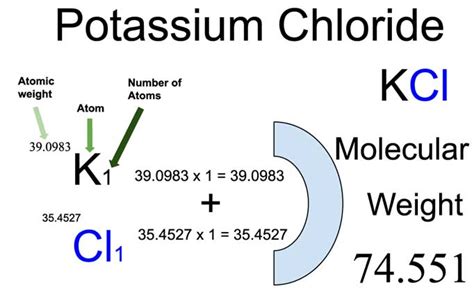
The molar mass of a substance is calculated by summing the atomic masses of each element present in the compound. For KCl, this involves considering the atomic masses of potassium (K) and chlorine (Cl).
Calculating the Molar Mass of KCl
To determine the molar mass of KCl, we need to refer to the periodic table, which provides the atomic masses of elements.
- Potassium (K) has an atomic mass of approximately 39.10 g/mol.
- Chlorine (Cl) has an atomic mass of approximately 35.45 g/mol.
Now, let’s calculate the molar mass of KCl:
- Molar mass of KCl = (Atomic mass of K) + (Atomic mass of Cl)
- Molar mass of KCl = (39.10 g/mol) + (35.45 g/mol)
- Molar mass of KCl = 74.55 g/mol
So, the molar mass of potassium chloride is approximately 74.55 grams per mole. This value is a crucial reference point for various calculations and applications involving KCl.
Applications of KCl Molar Mass
The molar mass of KCl finds extensive application in different scientific and industrial contexts. Here are some key areas where this information is vital:
Fertilizer Production
In agriculture, potassium chloride is a common component of fertilizers. The molar mass of KCl is used to determine the appropriate dosage and concentration of potassium in fertilizers. This ensures that crops receive the necessary nutrients without causing environmental harm due to over-application.
Medical Treatments
Potassium chloride is used in medical settings for various purposes, including treating potassium deficiency and as a cardiac medication. The molar mass of KCl is crucial for accurately calculating dosages and concentrations in medical formulations.
Environmental Science
KCl is used in environmental science for various applications, such as soil remediation and wastewater treatment. Understanding the molar mass of KCl helps researchers and scientists determine the appropriate quantities needed for effective treatment processes.
Chemical Reactions
In chemical reactions, the molar mass of KCl is essential for calculating reaction stoichiometry, determining reaction yields, and balancing chemical equations. This information is vital for ensuring the success and safety of chemical processes.
Quality Control and Analysis
Molar mass data is crucial for quality control and analysis in various industries. For example, in food production, KCl is used as a salt substitute and flavor enhancer. The molar mass helps ensure that the correct amounts of KCl are used, maintaining product quality and safety.
Conclusion
The molar mass of KCl, at approximately 74.55 g/mol, is a fundamental property with wide-ranging applications. From agriculture to medicine and environmental science, this value is a key reference point for professionals across diverse fields.
Understanding and applying molar mass concepts are essential for anyone working with KCl or similar compounds. This knowledge enables accurate calculations, ensures safe and effective applications, and contributes to the overall advancement of scientific and industrial practices.
For further exploration of molar mass concepts and their applications, readers can delve into the rich body of scientific literature available on the subject.