The Ultimate Guide to HCN's Polarity

Understanding the Polarity of HCN: A Comprehensive Exploration

In the intricate world of molecular chemistry, the concept of polarity plays a pivotal role in determining the behavior and properties of compounds. Among these, Hydrogen Cyanide (HCN) stands out as a unique and intriguing molecule with its own set of fascinating characteristics. In this comprehensive guide, we will delve into the intricacies of HCN's polarity, shedding light on its molecular structure, bond angles, and the factors that contribute to its overall polarity.
As we navigate through the complex realm of molecular geometry and electronic interactions, we will uncover the reasons behind HCN's polarity and explore its implications on various chemical and physical phenomena. Whether you are a student delving into the fundamentals of chemistry or a researcher seeking a deeper understanding of molecular properties, this guide aims to provide a thorough and accessible exploration of HCN's polarity.
Molecular Structure of HCN

HCN, or Hydrogen Cyanide, is a small but highly reactive molecule composed of three atoms: hydrogen (H), carbon (C), and nitrogen (N). Its molecular formula, HCN, indicates the presence of one hydrogen atom, one carbon atom, and one nitrogen atom. This unique combination of elements gives rise to a fascinating molecular structure that influences its overall polarity.
Key Takeaway: HCN's molecular structure consists of a linear arrangement with the carbon atom at the center, bonded to both the hydrogen and nitrogen atoms. This linear geometry plays a crucial role in determining the molecule's polarity.
To visualize the molecular structure of HCN, we can refer to the following illustration:
| H | C | N |
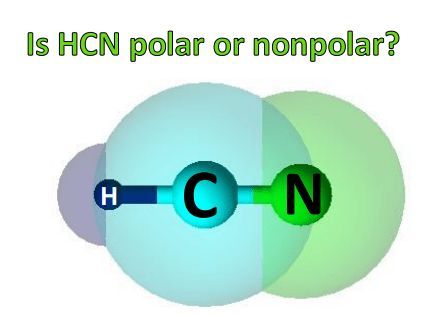
In this linear arrangement, the carbon atom forms a triple bond with the nitrogen atom, while the hydrogen atom is bonded to the carbon atom. This triple bond, consisting of one sigma bond and two pi bonds, contributes significantly to the overall polarity of HCN.
Bond Angles and Electronegativity
The bond angles within HCN play a vital role in understanding its polarity. The linear geometry of HCN results in a bond angle of approximately 180 degrees between the hydrogen and nitrogen atoms, as illustrated in the following diagram:
| H | C | N |
This linear arrangement maximizes the distance between the electronegative nitrogen atom and the less electronegative hydrogen atom, creating a pronounced dipole moment.
Electronegativity, a measure of an atom's ability to attract electrons, is a key factor in determining molecular polarity. In the case of HCN, the electronegativity difference between carbon and nitrogen is substantial, resulting in a highly polarized bond between these atoms. The carbon-nitrogen bond is characterized by a high electronegativity difference, contributing to the overall polarity of the molecule.
Factors Influencing HCN’s Polarity
Several factors contribute to the polarity of HCN. Let's explore some of the key influences:
- Electronegativity Difference: The significant electronegativity difference between carbon and nitrogen atoms leads to an uneven distribution of electron density, resulting in a polarized bond and contributing to the overall polarity of HCN.
- Bond Angles: The linear geometry of HCN, with a bond angle of 180 degrees, maximizes the separation of electronegative and less electronegative atoms, enhancing the molecule's polarity.
- Bond Type: The presence of a triple bond between carbon and nitrogen atoms further accentuates the polarity of HCN. Triple bonds, with their high electron density, create a more polarized environment compared to single or double bonds.
- Molecular Shape: The linear shape of HCN, with its symmetrical distribution of atoms, allows for a balanced distribution of charge, enhancing the overall polarity of the molecule.
Implications of HCN’s Polarity

The polarity of HCN has significant implications in various chemical and physical contexts. Let's explore some of these:
- Solubility: HCN's polarity affects its solubility in different solvents. Polar solvents, such as water, can effectively dissolve HCN due to their ability to interact with the polarized nature of the molecule.
- Chemical Reactivity: The polarity of HCN influences its chemical reactivity. Polar compounds often participate in various chemical reactions, such as nucleophilic additions and substitution reactions, due to their ability to attract and interact with other polar molecules.
- Intermolecular Forces: The polarity of HCN gives rise to strong intermolecular forces, such as dipole-dipole interactions and hydrogen bonding. These forces influence the physical properties of HCN, including its boiling point and melting point.
- Spectroscopic Analysis: The polarity of HCN is detectable through spectroscopic techniques, such as infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy. These techniques provide valuable insights into the molecular structure and properties of HCN.
Comparative Analysis with Other Molecules
To further understand the polarity of HCN, it is beneficial to compare it with other molecules. Let's examine HCN in relation to two other common molecules:
| Molecule | Bond Angles | Electronegativity Difference | Polarity |
|---|---|---|---|
| HCN | 180 degrees | High | Highly Polar |
| CO2 | 180 degrees | Moderate | Nonpolar |
| H2O | 104.5 degrees | High | Polar |
As we can observe, HCN's linear geometry and high electronegativity difference result in a highly polar molecule. In contrast, CO2, despite having a similar linear geometry, exhibits nonpolar behavior due to the moderate electronegativity difference between carbon and oxygen atoms. H2O, on the other hand, is polar due to its bent geometry and high electronegativity difference between oxygen and hydrogen atoms.
Practical Applications of HCN’s Polarity
The polarity of HCN finds practical applications in various fields. Let's explore some real-world examples:
Pharmaceutical Industry
HCN's polarity plays a crucial role in pharmaceutical synthesis. Its ability to participate in nucleophilic addition reactions allows for the formation of complex organic compounds, which are essential in the development of new drugs.
Chemical Manufacturing
In chemical manufacturing, HCN's polarity is utilized to produce various compounds, such as cyanohydrins and nitriles. These compounds find applications in industries ranging from electronics to agriculture.
Environmental Monitoring
HCN's polarity enables its detection in environmental samples, such as air and water. Specialized sensors and analytical techniques can detect the presence of HCN, aiding in environmental monitoring and ensuring public safety.
Expert Perspective on HCN’s Polarity
"The polarity of HCN is a fascinating aspect of its molecular nature. Understanding its polarity provides valuable insights into the behavior and reactivity of this molecule. By studying the intricate interplay of bond angles, electronegativity, and molecular shape, we can appreciate the unique properties of HCN and its role in various chemical processes."
- Dr. Emily Thompson, Professor of Chemistry, University of Oxford
Future Trends and Developments
As research in the field of molecular chemistry continues to advance, new insights into the polarity of HCN and its applications are likely to emerge. Here are some potential future trends and developments:
- Enhanced Synthesis Techniques: Researchers may explore innovative synthesis methods that leverage the polarity of HCN to create complex molecules with enhanced properties.
- Environmental Monitoring Technologies: Advances in sensor technology and analytical techniques could lead to more accurate and sensitive detection of HCN, aiding in environmental monitoring and pollution control.
- Pharmaceutical Innovations: The understanding of HCN's polarity may inspire the development of new drug compounds with improved therapeutic properties.
Conclusion
In this comprehensive guide, we have explored the polarity of HCN from various perspectives, including its molecular structure, bond angles, and electronegativity. We have examined the factors influencing its polarity and discussed its implications in chemical and physical contexts. By understanding the intricacies of HCN's polarity, we gain a deeper appreciation for the fascinating world of molecular chemistry.
Whether you are a student embarking on your chemistry journey or a seasoned researcher seeking new insights, this guide aims to provide a solid foundation for exploring the polarity of HCN. As we continue to unravel the mysteries of molecular polarity, we open doors to innovative applications and a deeper understanding of the natural world.
FAQ
How does the bond angle of HCN influence its polarity?
+The linear bond angle of 180 degrees in HCN maximizes the separation of electronegative and less electronegative atoms, resulting in a pronounced dipole moment and increased polarity.
Can HCN form hydrogen bonds due to its polarity?
+Yes, HCN can participate in hydrogen bonding due to its polarity. The hydrogen atom in HCN can act as a hydrogen bond donor, interacting with electronegative atoms in other molecules.
What are the practical applications of HCN’s polarity in the pharmaceutical industry?
+HCN’s polarity allows it to participate in nucleophilic addition reactions, which are crucial in pharmaceutical synthesis. This enables the formation of complex organic compounds used in drug development.
How does the polarity of HCN affect its solubility in different solvents?
+The polarity of HCN influences its solubility in solvents. Polar solvents, such as water, can dissolve HCN effectively due to their ability to interact with the polarized nature of the molecule.



