5 Ways to Understand BF3's Polarity

The concept of polarity is fundamental in chemistry, especially when dealing with molecular compounds like Boron Trifluoride (BF3). Polarity influences various chemical properties and behaviors, making it crucial for anyone studying or working with chemical compounds to grasp this concept. Here, we explore five effective methods to comprehend and determine the polarity of BF3.
1. Electronegativity Difference
The first step in understanding the polarity of any molecular compound is to examine the electronegativity values of its constituent atoms. Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. In the case of BF3, you can compare the electronegativity values of boron (B) and fluorine (F). Fluorine has a significantly higher electronegativity (3.98) compared to boron (2.04). This substantial difference indicates a high potential for electronegativity imbalance, a key factor in determining molecular polarity.
2. Lewis Structures and Bonding
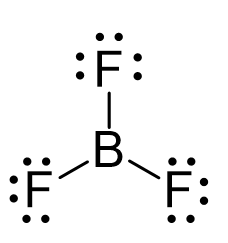
Drawing the Lewis structure of BF3 provides a visual representation of its molecular geometry and bonding patterns. In a Lewis structure, you can see the distribution of electrons in the molecule, including shared and unshared electron pairs. BF3 has a trigonal planar geometry, with three fluorine atoms surrounding a central boron atom. The Lewis structure reveals that each fluorine atom shares one electron with the boron atom, forming three single bonds. However, boron’s unfilled p-orbital can accommodate an additional electron, which can lead to the formation of a coordinate covalent bond with another molecule.
3. Molecular Geometry and Symmetry
The molecular geometry of BF3, with its trigonal planar structure, is highly symmetrical. Symmetry plays a crucial role in determining molecular polarity. In BF3, the three fluorine atoms are evenly spaced around the central boron atom, creating a perfect triangular arrangement. This symmetry results in a balanced distribution of electron density, which in turn influences the overall polarity of the molecule. While each individual bond between boron and fluorine is polar, the overall molecule has a non-polar nature due to its symmetrical geometry.
4. Dipole Moments
Dipole moments are vector quantities that describe the separation of positive and negative charges within a molecule. They are calculated based on the difference in electronegativity between bonded atoms and the distance between them. In BF3, although each B-F bond is polar due to the electronegativity difference, the dipole moments cancel each other out because of the molecule’s symmetrical arrangement. This cancellation of dipole moments contributes to the non-polar nature of BF3 as a whole.
5. Experimental Evidence and Spectroscopy

Experimental techniques, such as infrared (IR) spectroscopy, can provide valuable insights into the polarity of molecular compounds. IR spectroscopy measures the absorption of infrared radiation by molecules, which is influenced by the presence of polar bonds. In the case of BF3, IR spectroscopy reveals the absence of a strong absorption band typically associated with polar molecules. This experimental evidence further supports the conclusion that BF3 is a non-polar molecule, despite the presence of polar B-F bonds.
Conclusion
Understanding the polarity of BF3 involves a multifaceted approach, considering factors such as electronegativity differences, Lewis structures, molecular geometry, dipole moments, and experimental evidence. By examining these aspects, we can conclude that while each B-F bond in BF3 is polar, the overall molecule exhibits non-polar characteristics due to its symmetrical geometry and the cancellation of individual bond dipoles. This understanding of polarity is essential for predicting the behavior of BF3 in various chemical reactions and processes.
BF3 serves as an excellent example of how polarity can be influenced by both individual bond characteristics and overall molecular symmetry, offering a deeper insight into the fascinating world of molecular polarity.
What is the main factor that determines the polarity of a molecule like BF3?
+The primary factor that determines the polarity of a molecule like BF3 is the electronegativity difference between the bonded atoms. In the case of BF3, the significant difference in electronegativity between boron and fluorine leads to polar bonds. However, the symmetrical arrangement of these polar bonds results in a cancellation of their individual dipole moments, giving BF3 an overall non-polar character.
How does the molecular geometry of BF3 contribute to its polarity (or lack thereof)?
+The molecular geometry of BF3, with its trigonal planar structure, plays a crucial role in determining its polarity. The symmetrical arrangement of the three fluorine atoms around the central boron atom leads to a balanced distribution of electron density. This symmetry results in the cancellation of individual bond dipoles, making BF3 a non-polar molecule despite the presence of polar B-F bonds.
Can you explain the concept of dipole moments and their role in determining molecular polarity using BF3 as an example?
+Dipole moments are a measure of the separation of positive and negative charges within a molecule. In BF3, although each B-F bond is polar due to the electronegativity difference, the dipole moments of these bonds cancel each other out because of the molecule’s symmetrical arrangement. This cancellation of dipole moments contributes to the overall non-polar nature of BF3.
How does experimental evidence, such as IR spectroscopy, support the non-polar nature of BF3?
+IR spectroscopy measures the absorption of infrared radiation by molecules, which is influenced by the presence of polar bonds. In the case of BF3, IR spectroscopy reveals the absence of a strong absorption band typically associated with polar molecules. This experimental evidence supports the conclusion that BF3 is a non-polar molecule, as it does not exhibit the characteristic absorption patterns of polar compounds.
Are there any practical implications of understanding the polarity of BF3 in real-world applications?
+Yes, understanding the polarity of BF3 has practical implications in various fields. For example, in the chemical industry, BF3 is used as a catalyst in the production of various organic compounds. Knowing its polarity helps chemists predict its behavior in different chemical reactions and ensures the safe and efficient use of this compound in industrial processes.