Unveiling Ionic Bonds: Common Examples
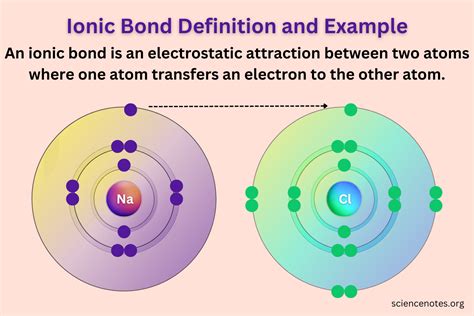
Ionic bonds are a fascinating and essential concept in chemistry, playing a pivotal role in the formation of various compounds. This type of chemical bonding occurs when atoms exchange electrons, leading to the formation of ions with opposite charges, which then attract each other, forming stable compounds. In this article, we will explore some common examples of ionic bonds, shedding light on their significance and the compounds they create.
The Basics of Ionic Bonding

Ionic bonding is a fundamental process in chemistry, and it’s rooted in the behavior of atoms. Atoms, being electrically neutral, strive for stability by achieving a full outer electron shell. This is where the concept of the octet rule comes into play—atoms tend to gain, lose, or share electrons to reach a stable electron configuration similar to that of a noble gas, with a full valence shell.
When two atoms with significantly different electronegativity values interact, one of them tends to completely lose one or more electrons, becoming a positively charged ion (cation), while the other gains those electrons, forming a negatively charged ion (anion). These ions are then attracted to each other due to their opposite charges, resulting in the formation of an ionic compound.
Think of ionic bonding as a contract between atoms—one atom agrees to give up some of its freedom (electrons) in exchange for stability, while the other gains that freedom but also gains a commitment (a positive charge) from the first atom.
Common Examples of Ionic Bonds
Sodium Chloride (NaCl)
One of the most well-known and ubiquitous examples of ionic bonding is sodium chloride, or table salt. This compound is formed when sodium (Na) loses an electron to become a cation (Na+), and chlorine (Cl) gains that electron to become an anion (Cl-). The positively charged sodium ions are then attracted to the negatively charged chlorine ions, forming a crystal lattice structure that gives table salt its solid form.
Sodium chloride serves as an excellent illustration of ionic bonding because it's a simple compound with a 1:1 ratio of cations to anions, making its structure easy to visualize and understand.
Magnesium Oxide (MgO)
Magnesium oxide is another classic example of an ionic compound. Here, magnesium (Mg) loses two electrons to become a cation (Mg2+), while oxygen (O) gains those two electrons to become an anion (O2-). The strong electrostatic attraction between these oppositely charged ions results in a highly stable compound.
Pros of MgO
- High melting point, making it useful in high-temperature applications.
- Good electrical insulator.
Cons of MgO
- Not soluble in water, limiting its biological and environmental applications.
- Can be abrasive, so it's not suitable for certain industrial processes.
Calcium Carbonate (CaCO3)
Calcium carbonate is a versatile compound found in a wide range of natural and industrial contexts. It’s formed through the ionic bonding of calcium (Ca) and carbonate (CO32-). Calcium loses two electrons to become a cation (Ca2+), while carbonate gains those two electrons and two more to form an anion. This compound is notable for its stability and the variety of forms it can take, from limestone to chalk.
Formation of Calcium Carbonate
- Calcium loses two electrons to become Ca2+.
- Carbonate gains those two electrons, plus two more, to form CO32-.
- The positively charged Ca2+ ions and negatively charged CO32- ions attract each other, forming the ionic compound.
Potassium Iodide (KI)
Potassium iodide is a crucial compound in medicine, particularly in the treatment of thyroid conditions. It’s formed through the ionic bonding of potassium (K) and iodine (I). Potassium loses an electron to become a cation (K+), and iodine gains that electron to become an anion (I-). This compound is soluble in water, which is a key property for its medicinal applications.
Why is potassium iodide important in thyroid health?
+Potassium iodide is crucial in thyroid health because it helps regulate the production of thyroid hormones. In the event of a nuclear emergency, radioactive iodine can be released, which can be harmful to the thyroid gland. Taking potassium iodide tablets can prevent the thyroid gland from absorbing this radioactive iodine, thus protecting it from potential damage.
Aluminum Oxide (Al2O3)
Aluminum oxide, also known as alumina, is a highly stable compound due to its strong ionic bonding. Aluminum loses three electrons to become a cation (Al3+), while oxygen gains those three electrons to become an anion (O2-). This compound is known for its hardness and high melting point, making it valuable in various industrial applications, including as an abrasive and a component in ceramics.
| Property | Description |
|---|---|
| Hardness | One of the hardest materials known, making it excellent for abrasive applications. |
| High Melting Point | Melts at an extremely high temperature, over 2000°C, making it suitable for high-temperature environments. |
| Insulator | A good electrical insulator, often used in electronic components. |
Applications of Ionic Compounds
Ionic compounds, formed through ionic bonding, have a wide range of applications across various industries:
- Medicine: As seen with potassium iodide, ionic compounds can play a critical role in healthcare, whether it’s for treatment or prevention.
- Construction: Materials like cement, which is primarily calcium silicate hydrate, are essential in construction due to their strength and durability.
- Electronics: Certain ionic compounds are used as insulators or components in electronic devices due to their electrical properties.
- Nutrition: Many ionic compounds are essential nutrients, such as calcium and potassium, which are crucial for various bodily functions.
- Environmental: Some ionic compounds are used in environmental remediation, like the use of aluminum sulfate to treat wastewater.
Conclusion
Ionic bonds are fundamental to the formation of many common and essential compounds. By understanding the principles of ionic bonding and exploring examples like sodium chloride, magnesium oxide, and calcium carbonate, we gain insight into the building blocks of our world. These compounds, with their unique properties and applications, demonstrate the power and versatility of ionic bonding in chemistry.



