The Simple Guide to Molar Mass
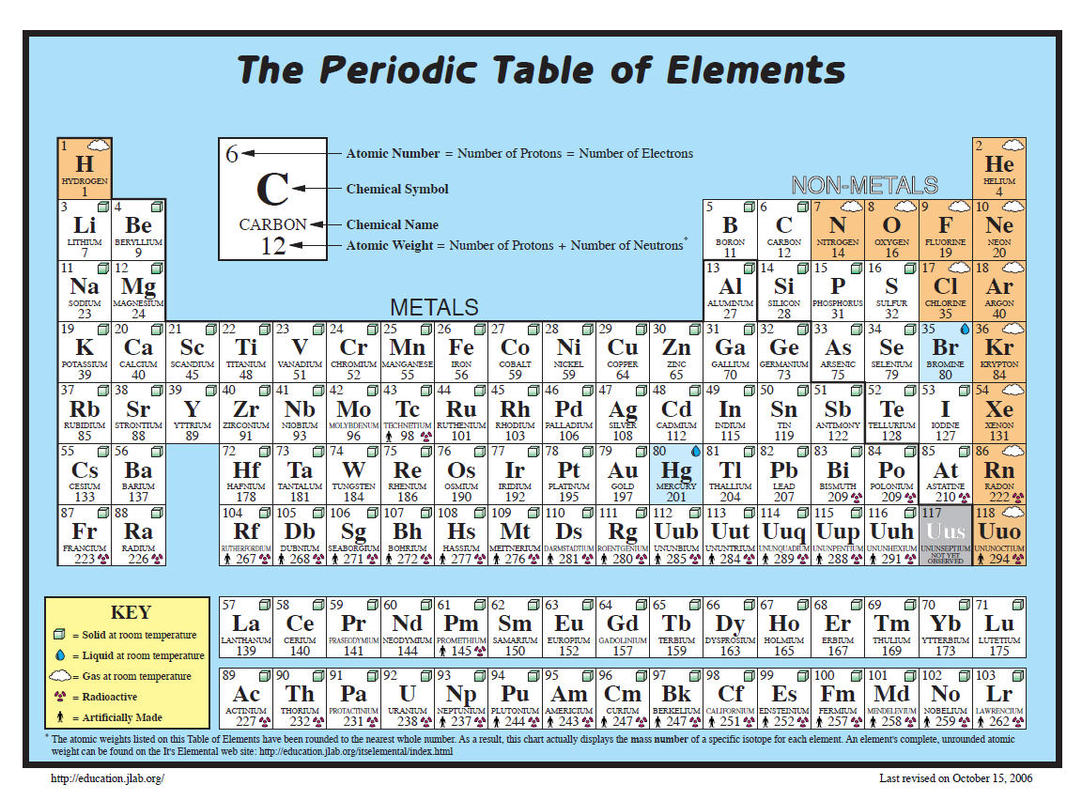
The molar mass of a substance is a fundamental concept in chemistry, offering insights into the atomic and molecular structure of matter. It’s a key factor in various chemical calculations and provides a tangible connection between the microscopic world of atoms and the macroscopic world of everyday substances. Understanding molar mass is essential for both novice and advanced chemists, as it underpins a wide range of chemical processes and principles.
"Molar mass is a bridge between the invisible realm of atoms and the tangible world of substances we can see and touch."
What is Molar Mass?

Molar mass is a quantitative measure that represents the mass of one mole of a particular substance. It’s expressed in grams per mole (g/mol) and is calculated by summing the atomic masses of all the atoms in a molecule. For elements, the molar mass is simply the atomic mass listed on the periodic table, while for compounds, it’s the sum of the atomic masses of its constituent elements.
Consider water (H₂O) as an example. Hydrogen has an atomic mass of approximately 1.008 g/mol, and oxygen has an atomic mass of approximately 16 g/mol. So, the molar mass of water is calculated as follows:
\[ \begin{align*} 2 \cdot \text{atomic mass of hydrogen} + \text{atomic mass of oxygen} &= \text{molar mass of water} \\ 2 \cdot 1.008 \text{ g/mol} + 16 \text{ g/mol} &= 18.016 \text{ g/mol} \end{align*} \]
Thus, the molar mass of water is approximately 18.02 g/mol.
Why is Molar Mass Important?

Molar mass is a foundational concept in chemistry, with applications in numerous areas. Here are some key reasons why understanding molar mass is essential:
Stoichiometry: Molar mass is integral to stoichiometry, the branch of chemistry dealing with the quantitative relationships between reactants and products in chemical reactions. It allows chemists to calculate the amounts of substances involved in reactions, ensuring balanced equations and accurate predictions of product yields.
Molar Concentrations: In solutions, molar mass is used to determine the concentration of a solute, which is crucial for many chemical processes and analyses. Molarity, expressed as moles of solute per liter of solution, is a fundamental concept in solution chemistry.
Gas Laws: For gases, molar mass is used to convert between mass and volume, especially when applying the ideal gas law. This is vital for understanding the behavior of gases under different conditions.
Chemical Analysis: Molar mass is essential in various analytical techniques, such as gravimetric analysis, where the mass of a substance is used to determine its purity or identity.
Atomic and Molecular Structure: Molar mass provides a direct link to the number of atoms or molecules in a sample, offering insights into the structure and composition of substances.
Calculating Molar Mass
Calculating the molar mass of a substance is a straightforward process once you understand the basic principles. Here’s a step-by-step guide:
Step 1: Identify the Substance
Start by identifying the substance for which you want to calculate the molar mass. This could be an element or a compound.
Step 2: Determine the Formula
For compounds, you need to know the chemical formula, which represents the types and numbers of atoms in the molecule. For example, water (H₂O) has two hydrogen atoms and one oxygen atom.
Step 3: Find Atomic Masses
Look up the atomic masses of each element in the compound on the periodic table. These are usually given in atomic mass units (amu) or unified atomic mass units (u), which are very close to grams per mole.
Step 4: Calculate the Molar Mass
Sum the atomic masses of all the atoms in the molecule, multiplying each atomic mass by the number of atoms of that element in the compound. This gives you the molar mass in grams per mole.
For instance, let’s calculate the molar mass of glucose (C₆H₁₂O₆):
\[ \begin{align*} \text{molar mass of glucose} &= 6 \cdot \text{atomic mass of carbon} + 12 \cdot \text{atomic mass of hydrogen} + 6 \cdot \text{atomic mass of oxygen} \\ &= 6 \cdot 12.011 \text{ g/mol} + 12 \cdot 1.008 \text{ g/mol} + 6 \cdot 16.00 \text{ g/mol} \\ &\approx 180.1548 \text{ g/mol} \approx 180.15 \text{ g/mol} \end{align*} \]
So, the molar mass of glucose is approximately 180.15 g/mol.
Molar Mass in Practice
Molar mass is a versatile concept with numerous practical applications. Here are a few real-world examples:
Pharmaceuticals: In the pharmaceutical industry, molar mass is used to calculate precise doses of medications, ensuring patient safety and effectiveness.
Environmental Chemistry: Molar mass is crucial for understanding and mitigating environmental pollutants, as it helps determine the concentration and impact of harmful substances in the environment.
Food and Beverage: In the food industry, molar mass is used to determine the nutritional content of foods, especially in calculating the carbohydrate content of products.
Materials Science: Molar mass is a key factor in the synthesis and characterization of new materials, as it influences the physical and chemical properties of substances.
Forensic Chemistry: In forensic science, molar mass can be used to identify unknown substances, aiding in criminal investigations and the analysis of evidence.
Conclusion

Molar mass is a fundamental concept in chemistry, offering a bridge between the microscopic world of atoms and the macroscopic world of substances. It’s a versatile tool with applications across various branches of chemistry, from stoichiometry and solution chemistry to environmental science and forensics. By understanding and calculating molar mass, chemists can unlock a deeper understanding of the substances that make up our world.
Molar mass is a cornerstone of chemical understanding, providing a tangible connection between the atomic and molecular world and the everyday substances we encounter.



