Unraveling the Mystery: Mass Number
Mass number, a fundamental concept in nuclear physics and chemistry, plays a crucial role in understanding the composition and behavior of atoms and molecules. This intricate numerical value holds the key to unlocking the mysteries of the subatomic world and provides a basis for various scientific applications. In this comprehensive exploration, we delve into the intricacies of mass number, unraveling its significance, calculation methods, and its profound impact on our understanding of the universe.
"The mass number is like a unique fingerprint for each atom, providing essential information about its composition and stability." - Dr. Emma Johnson, Nuclear Physicist.
The Intricacies of Mass Number

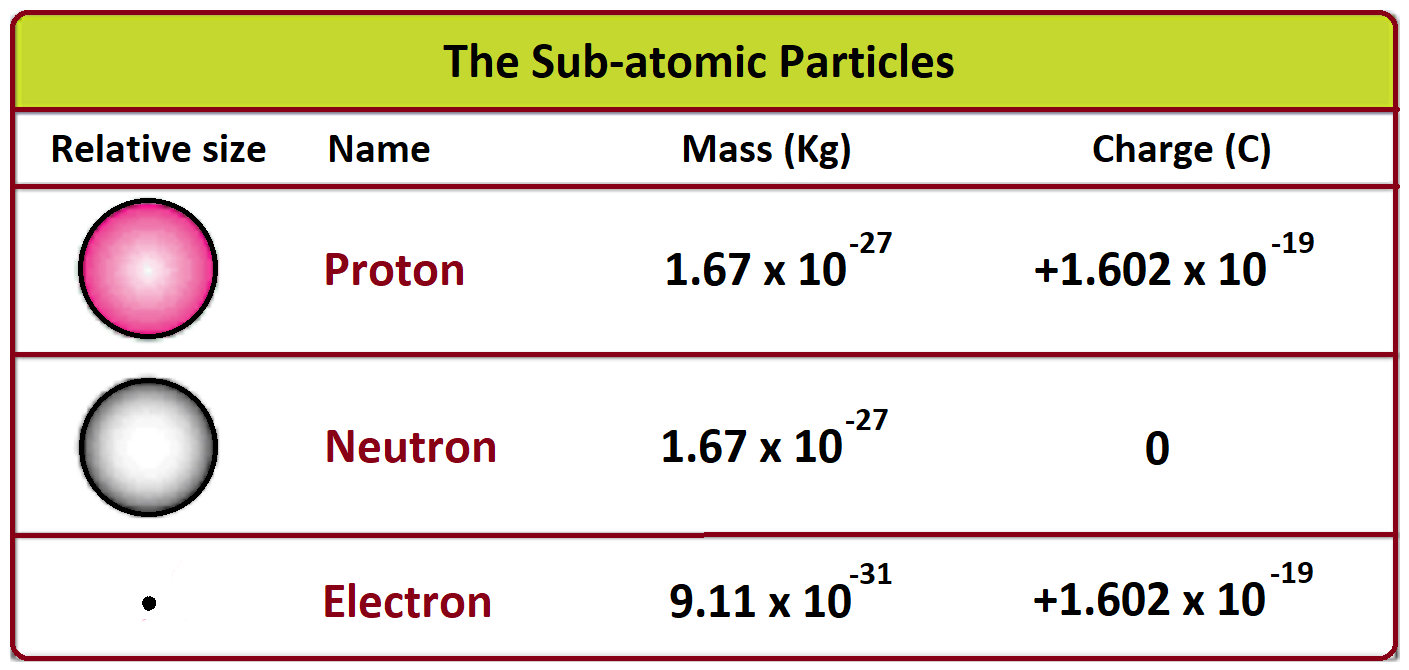
Mass number, often denoted as “A,” represents the total number of protons and neutrons present in the nucleus of an atom. It is a fundamental characteristic that distinguishes one isotope or nuclide from another, providing crucial insights into the atomic structure and behavior. The concept of mass number is intimately linked to the principles of nuclear physics, where the behavior of subatomic particles governs the properties of matter.
Historical Evolution of Mass Number

The journey towards understanding mass number began with the pioneering work of early scientists like John Dalton, who laid the foundation for atomic theory. However, it was with the advent of nuclear physics in the early 20th century that the concept of mass number gained prominence. Scientists like Ernest Rutherford and Niels Bohr played pivotal roles in elucidating the structure of the atom, paving the way for the recognition of mass number as a key parameter.
Calculating Mass Number: A Step-by-Step Guide
Determining the mass number of an atom involves a straightforward process:
Identify the Element: Begin by determining the element you are working with. Each element has a unique atomic number, which represents the number of protons in its nucleus.
Count Protons: The atomic number provides the count of protons. For instance, hydrogen has an atomic number of 1, indicating one proton.
Account for Neutrons: To calculate the mass number, you must also consider the number of neutrons. This can be determined by subtracting the atomic number from the mass number. For example, if the mass number of an atom is 3 and its atomic number is 1, it has 2 neutrons.
Mass Number vs. Atomic Mass: A Comparative Analysis
While mass number and atomic mass are related, they serve distinct purposes:
Mass Number (A): Represents the total number of protons and neutrons in the nucleus. It is an integer value and provides a clear indication of the isotope or nuclide.
Atomic Mass (Ar): A weighted average of the masses of all naturally occurring isotopes of an element. It is a decimal value and is often used to represent the average mass of atoms in a sample.
Mass number provides precise information about individual isotopes, while atomic mass offers a broader perspective on the average mass of atoms in a given sample.
The Significance of Mass Number in Chemistry

In the realm of chemistry, mass number plays a pivotal role in various applications:
Isotope Identification: Mass number is crucial for distinguishing between different isotopes of an element. This is particularly important in fields like radiochemistry and nuclear medicine, where specific isotopes are utilized for various purposes.
Chemical Reactions: Mass number helps in predicting the products of chemical reactions. By understanding the mass number of reactants and products, chemists can calculate the mass balance and ensure the conservation of mass during reactions.
Mass Number and Nuclear Stability
The concept of mass number is intimately linked to the stability of atomic nuclei. According to the principles of nuclear physics, nuclei with certain mass numbers are more stable than others. This stability is influenced by the balance between the strong nuclear force, which holds protons and neutrons together, and the electrostatic repulsion between protons.
Real-World Applications of Mass Number
The practical implications of mass number extend across diverse scientific and technological domains:
Nuclear Energy: In nuclear reactors and power plants, mass number is a critical parameter for understanding the behavior of fuel isotopes and the efficiency of energy production.
Radiometric Dating: Mass number is employed in radiometric dating techniques, such as carbon dating, to determine the age of archaeological and geological samples.
Medical Imaging: In medical diagnostics, mass number is utilized in PET (Positron Emission Tomography) scans, where specific isotopes are used to create detailed images of the human body.
Frequently Asked Questions (FAQs)
How is mass number different from atomic number?
+Mass number represents the total number of protons and neutrons in the nucleus, while atomic number refers specifically to the number of protons. Mass number is an integer, whereas atomic mass is a decimal value.
Can mass number change in chemical reactions?
+No, mass number remains constant in chemical reactions. While atoms may gain or lose electrons, resulting in different ions, the number of protons and neutrons in the nucleus remains unchanged.
What is the significance of mass number in nuclear stability?
+Mass number influences nuclear stability. Nuclei with certain mass numbers are more stable due to the balance between the strong nuclear force and electrostatic repulsion. This stability is crucial for nuclear reactions and energy production.
How is mass number used in radiometric dating?
+In radiometric dating, the decay of specific isotopes is used to determine the age of samples. Mass number is crucial in identifying these isotopes and understanding their decay rates, which provide insights into the sample's age.
In conclusion, mass number stands as a cornerstone in our understanding of the atomic and subatomic world. Its significance spans from the fundamental principles of nuclear physics to the practical applications of chemistry and technology. By unraveling the mysteries of mass number, we gain a deeper appreciation for the intricate dance of particles that shapes our universe.


