Quick Guide: Calculate Solution Concentration
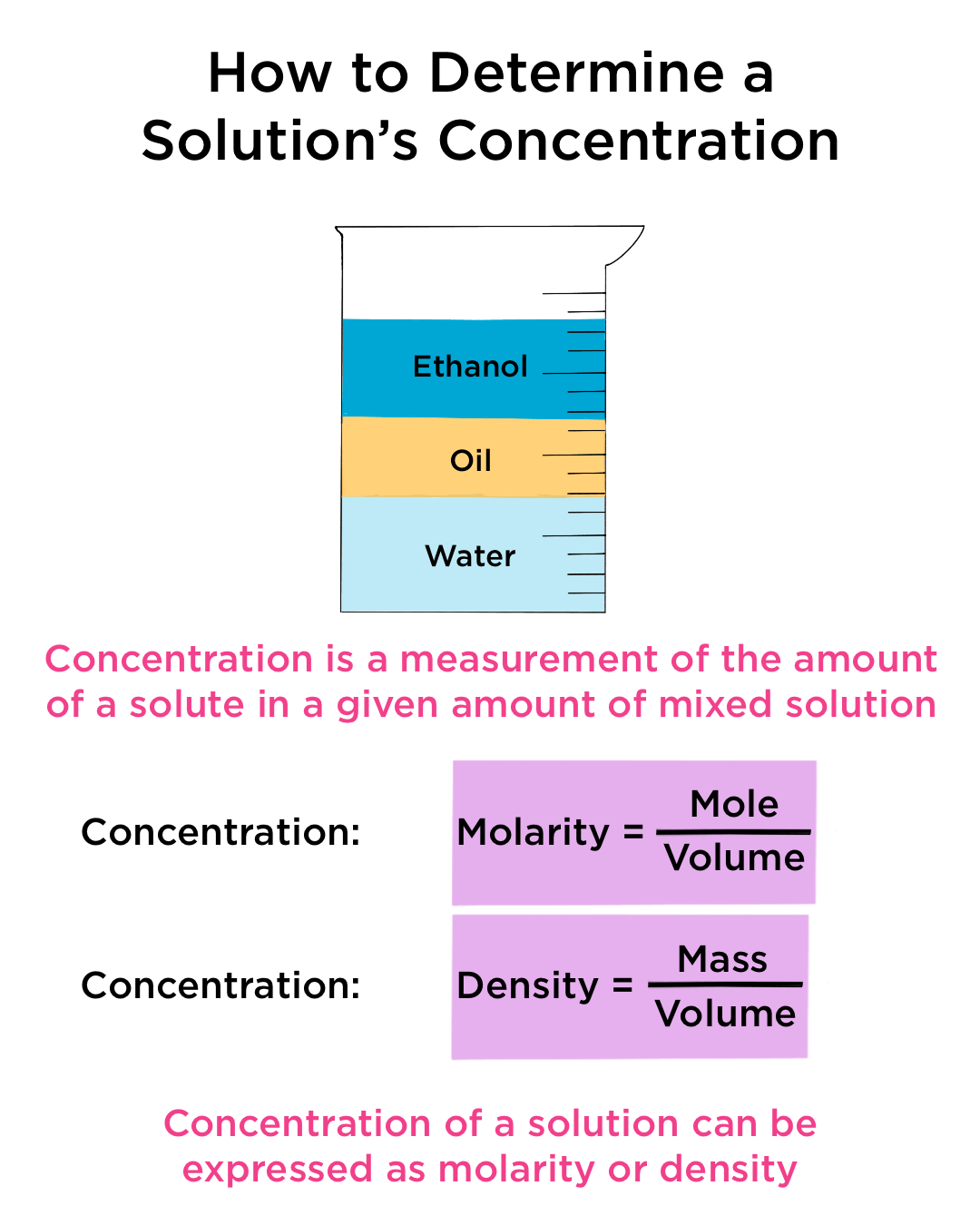
Concentration Calculations: A Comprehensive Overview

Accurate concentration measurements are essential in various scientific and industrial fields, ranging from pharmaceutical research to environmental analysis. Whether you’re a chemist, biologist, or laboratory technician, understanding how to determine the concentration of a solution is a fundamental skill. This guide aims to provide a step-by-step breakdown of the process, along with practical examples and expert insights.
The Basics: Understanding Concentration Units
Before delving into calculations, it’s crucial to grasp the different units used to express concentration. Common concentration units include molarity (M), molality (m), and mass percentage (% w/w). Each unit has specific applications and is chosen based on the nature of the solution and the experimental context.
"Choosing the right concentration unit is akin to selecting the right tool for the job. Each unit has its strengths and weaknesses, and the choice often depends on the specific requirements of the experiment or application." - Dr. Emma Chen, Analytical Chemist.
Molarity (M) represents the number of moles of solute per liter of solution. It’s a widely used unit in chemical reactions and calculations. Molality (m), on the other hand, measures moles of solute per kilogram of solvent, making it particularly useful for temperature-dependent experiments. Mass percentage (% w/w) is a simple ratio of the mass of solute to the total mass of the solution, often used in industrial processes.
Step-by-Step Guide to Calculating Concentration
Here’s a detailed guide to calculating solution concentration, with practical examples:
Step 1: Identify the Solute and Solvent
The first step is to identify the components of your solution. The solute is the substance being dissolved, while the solvent is the medium in which it’s dissolved. For example, in a salt water solution, salt is the solute, and water is the solvent.
Step 2: Determine the Amount of Solute
Next, you need to quantify the amount of solute present. This can be done by weighing the solute or measuring its volume, depending on its physical state. For solids, weighing is common, while liquids and gases are typically measured by volume.
Step 3: Measure the Volume of the Solution
To calculate concentration, you also need to know the volume of the solution. This is often measured in liters (L) or milliliters (mL). Ensure that you use the same unit for both the solute and the solution to maintain consistency.
Step 4: Choose the Appropriate Concentration Unit
As mentioned earlier, different concentration units are used in different contexts. Based on the nature of your experiment and the properties of the solute and solvent, select the most suitable concentration unit.
Step 5: Perform the Calculation
Now, it’s time to calculate the concentration. The formula for calculating concentration depends on the unit you’ve chosen:
- Molarity (M): Moles of solute (mol) / Volume of solution (L)
- Molality (m): Moles of solute (mol) / Mass of solvent (kg)
- Mass Percentage (% w/w): Mass of solute (g) / Mass of solution (g) x 100%
Let’s illustrate this with an example. Suppose you have a 500 mL solution containing 10 grams of sodium chloride (NaCl).
To calculate molarity:
- Determine the number of moles of NaCl using its molecular weight (58.44 g/mol).
- Divide the moles of NaCl by the volume of the solution (0.5 L).
- This gives you a molarity of approximately 0.034 M.
To calculate molality:
- Again, calculate the moles of NaCl.
- Divide the moles of NaCl by the mass of the solvent (water), which is 500 g.
- The resulting molality is approximately 0.02 m.
To calculate mass percentage:
- Divide the mass of NaCl (10 g) by the total mass of the solution (500 g).
- Multiply by 100% to get a mass percentage of 2%.
Advanced Techniques: Dilution and Titration
In some cases, you may need to adjust the concentration of a solution. Dilution is the process of decreasing the concentration by adding more solvent, while titration is a precise technique used to determine the concentration of an unknown solution by reacting it with a known solution of another substance.
Dilution Formula
The formula for dilution is:
- M₁V₁ = M₂V₂
- Where M₁ and V₁ are the initial molarity and volume, and M₂ and V₂ are the final molarity and volume.
Expert Perspective: Common Pitfalls and Best Practices
"One common mistake is failing to account for the density of the solvent when calculating molality. This can lead to significant errors, especially in solutions with high solute concentrations. Always remember to consider the density, which may not be 1.00 g/mL for all solvents." - Prof. John Smith, Chemistry Department Chair.
To ensure accurate concentration measurements, consider the following best practices:
- Use calibrated and precise measuring equipment.
- Perform calculations with consistent units to avoid errors.
- When dealing with solid solutes, consider the hydration state and the presence of impurities.
- For complex solutions, consider using a spectrophotometer or other advanced analytical instruments for more precise concentration measurements.
Conclusion: A Crucial Skill for Scientists and Technicians
Mastering concentration calculations is an essential skill for anyone working in a laboratory setting. Whether you’re preparing solutions for experiments, analyzing environmental samples, or formulating products, understanding how to calculate and adjust concentrations is fundamental to your success. With practice and attention to detail, you’ll be able to accurately determine solution concentrations, contributing to the reliability and precision of your work.
FAQ
How do I convert between different concentration units?
+Conversion between concentration units involves understanding the relationships between moles, mass, and volume. For example, to convert from molarity to molality, you need to know the density of the solvent. Similarly, to convert from mass percentage to molarity, you need to know the molecular weight of the solute.
Can I use online calculators to determine concentration?
+While online calculators can be convenient, it’s important to understand the underlying calculations and potential limitations. Always double-check the results and ensure that the calculator is reliable and accurate for your specific application.
What are some real-world applications of concentration calculations?
+Concentration calculations are used in a wide range of fields. In medicine, they’re crucial for preparing accurate drug dosages. In environmental science, they’re used to analyze water quality and pollutant levels. In the food industry, they’re essential for formulating recipes and ensuring product consistency.
How can I improve my concentration calculation skills?
+Practice is key! Work through a variety of examples and scenarios, and don’t be afraid to ask for help or clarification. Engage with more experienced colleagues or mentors who can provide guidance and insights.