Unveiling 5 Simple Ways to Find Atomic Mass
Atomic Mass: A Fundamental Property of Elements

Determining the atomic mass of an element is a crucial step in understanding its nature and behavior. While it may seem complex, especially for those new to the world of chemistry, the process can be broken down into simple, manageable steps.
In this guide, we’ll explore five straightforward methods that will help you uncover the atomic mass of any element, shedding light on a fundamental property that defines the very essence of matter.
Method 1: Using the Periodic Table
The periodic table is a powerful tool that organizes elements based on their atomic number and other properties. Each element’s position on the table provides valuable information, including its atomic mass.
To find the atomic mass using the periodic table, simply locate the element of interest and refer to the value given in the bottom left corner of its box. This number represents the average atomic mass of the element, taking into account the masses of its isotopes.
The periodic table is a chemist's best friend. It's not just a decorative wall poster; it's a roadmap to the universe of elements. Every element has its unique identity, and its position on the table tells a story of its atomic mass, among other fascinating characteristics.
Method 2: Averaging Isotope Masses
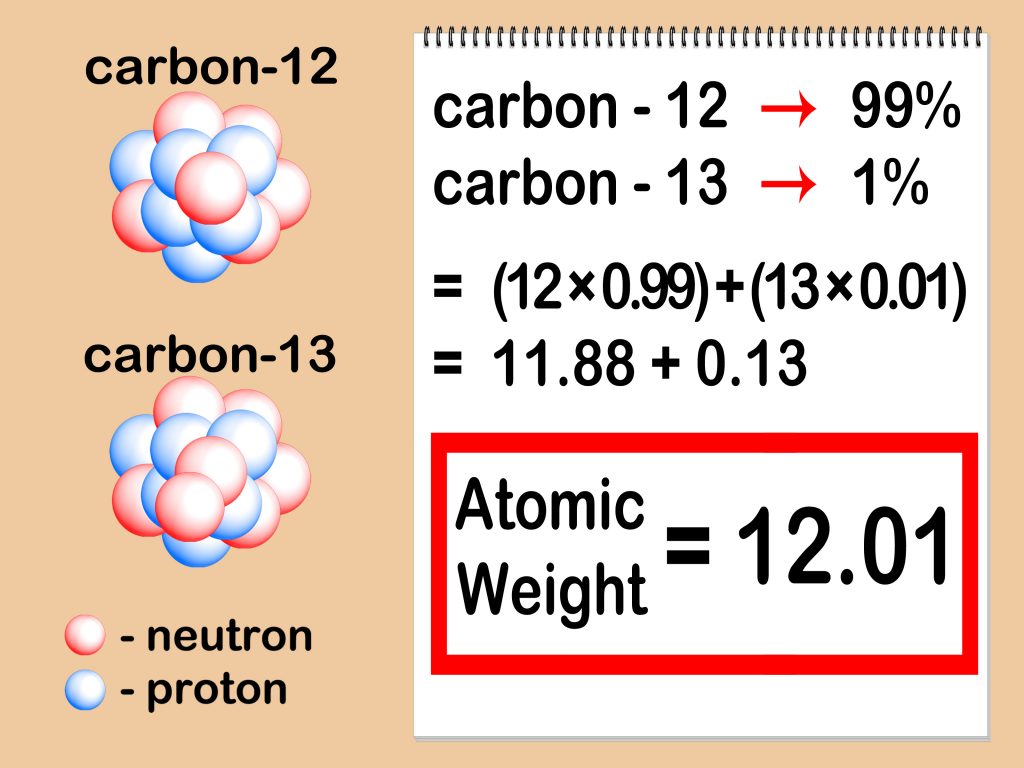
Elements often exist as different isotopes, which are atoms of the same element but with varying numbers of neutrons. Each isotope has its own atomic mass, and to find the average atomic mass, you need to calculate the weighted average of these masses.
Let’s take carbon as an example. Carbon has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. To find the average atomic mass of carbon:
- Identify the natural abundance of each isotope. Carbon-12 is the most abundant at 98.9%, while carbon-13 is 1.1% and carbon-14 is trace amounts.
- Multiply the abundance percentage by the mass number of each isotope. For carbon-12, it's 12 x 98.9% = 11.868. For carbon-13, it's 13 x 1.1% = 1.43.
- Sum up the values from step 2 to get the average atomic mass. For carbon, it's 11.868 + 1.43 = 13.3. This value is rounded to the nearest whole number, giving us an average atomic mass of 12 for carbon.
Method 3: Employing Mass Spectrometry
Mass spectrometry is a highly precise analytical technique used to determine the mass-to-charge ratio of ions. It can provide accurate measurements of atomic masses and is particularly useful for complex molecules and compounds.
In mass spectrometry, a sample is ionized, and the resulting ions are accelerated and passed through a magnetic field. The ions’ trajectories are then analyzed to determine their masses.
Mass spectrometry is like a detective, unraveling the secrets hidden within molecules. It can identify the atomic masses of elements with incredible precision, providing invaluable insights into the composition of substances.
Method 4: Using Online Calculators and Databases
In today’s digital age, numerous online resources and databases provide quick and convenient ways to find atomic masses. These tools can be especially useful when you need to compare and contrast the atomic masses of multiple elements or when you’re working with less common isotopes.
Some popular online calculators and databases include:
- WebElements
- The Periodic Table of the Elements
- PubChem
- NIST Atomic Weights and Isotopic Compositions
Method 5: Experimental Determination
For those with access to a laboratory, experimental determination of atomic mass can be a fascinating and hands-on approach. This method involves isolating a pure sample of the element and then using specialized equipment to measure its mass.
One common experimental technique is to use a mass spectrometer to directly measure the mass of individual atoms. This method provides highly accurate results but requires specialized training and equipment.
Pros
- Provides highly accurate results.
- Allows for hands-on learning and experimentation.
Cons
- Requires specialized equipment and training.
- May be time-consuming and resource-intensive.
Key Takeaway
Determining atomic mass is a fundamental skill in chemistry, and it’s easier than you might think. Whether you’re using the periodic table, calculating averages, employing mass spectrometry, utilizing online resources, or conducting experiments, each method provides a unique window into the atomic world.
Atomic mass is the foundation upon which we build our understanding of elements and their behavior. By mastering these simple methods, you unlock the secrets of the universe, one element at a time.
FAQ Section

Can I find the atomic mass of an element without a periodic table or online resources?
+While having a periodic table or online resources can be convenient, it’s not always necessary. You can calculate the average atomic mass by determining the natural abundance and mass of each isotope. This method requires some basic calculations but can be done without external references.
Why are atomic masses not always whole numbers?
+Atomic masses are not always whole numbers because they are averages that consider the masses of all isotopes of an element. Since isotopes have different masses, the average can be a decimal number. For example, hydrogen has an atomic mass of approximately 1.008, which is the average of the masses of its isotopes: protium (1), deuterium (2), and tritium (3)
Are there any limitations to the accuracy of atomic mass measurements?
+Yes, the accuracy of atomic mass measurements can be influenced by various factors, including the precision of the measuring equipment, the purity of the sample, and the presence of trace impurities. Mass spectrometry, for instance, can provide extremely accurate results, but its accuracy is dependent on the quality of the equipment and the expertise of the operator.
How do scientists decide which method to use for determining atomic mass?
+The choice of method depends on several factors, including the availability of resources, the element or compound of interest, and the desired level of accuracy. For routine analyses, the periodic table or online databases are often sufficient. When higher precision is required, experimental methods like mass spectrometry are employed.