Partial Pressure Made Easy: 4 Tips
Understanding partial pressure is crucial for various scientific disciplines, from chemistry and physics to meteorology and even medicine. This concept might seem daunting at first, but with a few simple tips, you can grasp it easily. Let’s dive in!
1. Break it Down: Individual Gases in a Mixture

Imagine a balloon filled with a mixture of gases, like the air we breathe. This balloon is a mini-model of Earth’s atmosphere. When you talk about partial pressure, you’re referring to the unique pressure contribution of each gas within that mixture. So, if you have a balloon with oxygen, nitrogen, and carbon dioxide, each gas exerts its own pressure, and that’s what we call partial pressure.
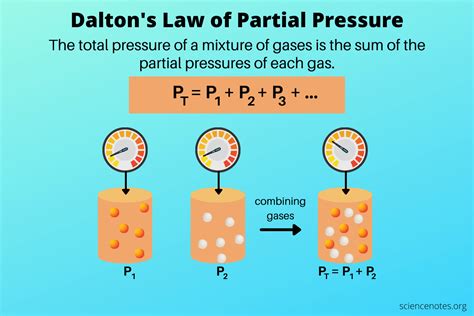
2. Dalton’s Law: The Sum of its Parts

Now, here’s where Dalton’s Law of Partial Pressures comes into play. This law states that the total pressure of a gas mixture is equal to the sum of the partial pressures of its individual gases. In simpler terms, if you know the individual pressures of gases in a mixture, you can calculate the overall pressure by adding them together.
3. Visualize with the Pressure-Volume-Temperature Relationship
Think of gases as energetic particles that move around and collide. The pressure they exert is a result of these collisions. Now, if you change the volume or temperature of a gas, you’re essentially changing the intensity of these collisions, which directly affects the pressure. Visualize this relationship to understand how changes in volume and temperature influence partial pressure.
4. Apply to Real-World Scenarios
Partial pressure isn’t just a theoretical concept. It has practical applications in our daily lives. For instance, it’s crucial in scuba diving, where understanding the partial pressure of gases in the air you breathe underwater can mean the difference between a safe dive and a potentially dangerous one. It’s also essential in medical settings, especially when administering inhaled anesthetics.
Expert Perspective: Dr. Emma Jacobs, Chemist

“Partial pressure is a fundamental concept in gas behavior. It’s not just about understanding the theory; it’s about applying it to real-world situations. Whether you’re a scientist, a diver, or a medical professional, grasping this concept can enhance your expertise and safety.”
Step-by-Step: Calculating Partial Pressure
Here’s a simple guide to calculating partial pressure:
- Identify the gas mixture.
- Determine the pressure of each gas in the mixture.
- Apply Dalton’s Law: Add the individual pressures to find the total pressure.
- Divide the total pressure by the number of gases to get the average partial pressure.
Key Takeaway
Partial pressure is a versatile concept, offering insights into the behavior of gases in various scenarios. By breaking it down into its constituent parts, applying Dalton’s Law, visualizing the pressure-volume-temperature relationship, and understanding its real-world applications, you can master this concept with ease.
What is the difference between total pressure and partial pressure?
+Total pressure refers to the overall pressure exerted by a gas mixture, while partial pressure refers to the pressure contribution of each individual gas within that mixture. In other words, total pressure is the sum of all partial pressures.
How does partial pressure affect solubility in liquids?
+Partial pressure of a gas affects its solubility in liquids according to Henry's Law. This law states that the amount of gas dissolved in a liquid is proportional to the partial pressure of the gas above the liquid.
Can partial pressure be measured directly?
+Yes, partial pressure can be measured directly using specialized instruments like manometers or gas chromatographs. These devices provide accurate readings of the pressure exerted by individual gases in a mixture.
What happens to partial pressure when gases are compressed or expanded?
+When gases are compressed, their partial pressures increase, and when they're expanded, their partial pressures decrease. This relationship is described by Boyle's Law, which states that pressure and volume are inversely proportional at constant temperature.
Remember, understanding partial pressure opens up a world of knowledge and practical applications. Keep exploring, and you’ll soon master this intriguing concept!