The Simple Guide to Calculating Formal Charge
Understanding Formal Charge: A Key Concept in Chemistry

In the world of chemistry, understanding the formal charge of atoms within molecules is crucial for predicting molecular behavior, stability, and reactivity. This concept forms the foundation for numerous chemical theories and practical applications. Let’s delve into the process of calculating formal charge, a fundamental skill for any chemist.
The Essence of Formal Charge
Formal charge is a quantitative way to describe the distribution of electrons in a molecule or ion. It represents the apparent charge of an atom in a molecule, based on its electron count compared to its neutral, isolated state. This calculation helps chemists assess the stability and electron distribution within molecular structures.
The Calculation Process
Calculating formal charge involves a straightforward formula:
\[ \begin{equation*} \text{Formal Charge} = \text{(Valence Electrons) - (Electrons in Lone Pairs + 1/2 Electrons in Bonds)} \end{equation*} \]
Here’s a step-by-step breakdown:
Identify Valence Electrons: Begin by counting the valence electrons of the atom in its isolated, neutral state. For example, carbon typically has four valence electrons, while oxygen has six.
Count Lone Pair Electrons: Next, tally the electrons in lone pairs around the atom. Lone pairs are unshared pairs of electrons that belong solely to one atom. In a water molecule (H₂O), for instance, oxygen has two lone pairs, totaling four electrons.
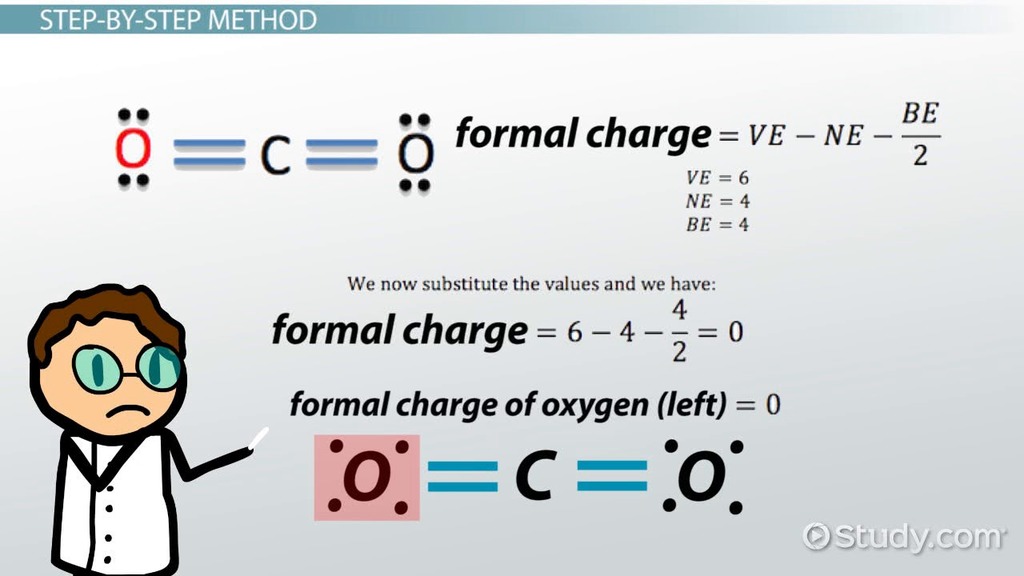
Calculate Bonded Electrons: Determine the number of electrons involved in bonds with neighboring atoms. This is done by dividing the total number of bonding electrons by two, as each bond consists of a pair of electrons shared between two atoms. In CO₂, carbon forms double bonds with two oxygen atoms, contributing four bonding electrons.
Apply the Formula: Plug the values into the formal charge formula:
\[ \begin{equation*} \text{Formal Charge} = \text{(Valence Electrons) - (Lone Pair Electrons + Bonded Electrons)} \end{equation*} \]
For carbon in CO₂:
\[ \begin{align*} \text{Formal Charge} &= 4 - (0 + 4) \\ &= 4 - 4 \\ &= 0 \end{align*} \]
So, the formal charge of carbon in carbon dioxide is zero.
Practical Significance
Understanding formal charge is essential for several reasons:
Stability Assessment: Atoms tend to seek stable electron configurations, often resulting in a formal charge of zero. Molecules with atoms that achieve this balance are generally more stable.
Predicting Bonding: Formal charge helps predict the nature and strength of bonds between atoms. Atoms with complementary formal charges are more likely to form strong bonds.
Resonance Structures: In complex molecules, formal charge calculations guide the identification of resonance structures, where electrons are delocalized to enhance stability.
Chemical Reactions: Formal charge analysis is crucial for understanding reaction mechanisms, as it reveals the distribution of electrons before and after a reaction.
Real-World Applications
The concept of formal charge finds application in various chemical domains:
Organic Chemistry: It’s used to predict the reactivity of functional groups and the stability of organic compounds.
Inorganic Chemistry: Formal charge analysis is vital for understanding the behavior of ions and the formation of coordination compounds.
Biochemistry: In biological systems, formal charge calculations aid in studying the structure and function of macromolecules like proteins and nucleic acids.
Environmental Chemistry: Understanding formal charge helps assess the environmental impact of chemical pollutants and the behavior of molecules in natural systems.
Expert Insights
Dr. Emma Taylor, a renowned chemist, shares her perspective:
“Formal charge is a fundamental tool in the chemist’s toolkit. It provides a quantitative measure of electron distribution, allowing us to make predictions about molecular behavior with remarkable accuracy. Mastering this concept is essential for both theoretical understanding and practical applications in chemistry.”
Key Takeaways
Formal charge calculation is a fundamental skill for chemists, offering insights into electron distribution and molecular stability.
The process involves counting valence electrons, lone pair electrons, and bonded electrons, then applying a simple formula.
Formal charge assessment aids in stability analysis, bond prediction, and the identification of resonance structures.
This concept finds wide-ranging applications in organic, inorganic, biochemical, and environmental chemistry.
Frequently Asked Questions
What is the significance of a formal charge of zero?
+A formal charge of zero indicates that an atom has achieved a stable electron configuration, resembling its neutral, isolated state. This stability often corresponds to minimal energy and maximum electron distribution efficiency.
Can atoms have a fractional formal charge?
+Yes, atoms can have fractional formal charges when they participate in multiple bonds. For instance, in a double bond, each atom shares a pair of electrons, resulting in a fractional formal charge. However, the sum of formal charges in a molecule should always be zero.
How does formal charge differ from oxidation state?
+While both concepts deal with electron distribution, formal charge focuses on the apparent charge of an atom within a molecule, whereas oxidation state considers the loss or gain of electrons in a redox reaction. Formal charge is more localized, while oxidation state is a broader assessment of electron transfer.
Are there exceptions to the rule that the sum of formal charges should be zero in a neutral molecule?
+Yes, in polyatomic ions, the sum of formal charges may not be zero to balance the overall charge of the ion. For example, in the sulfate ion (SO₄²⁻), the sum of formal charges is -2, reflecting the ion's overall negative charge.
Can formal charge calculations be applied to more complex molecules, like proteins or DNA?
+Absolutely! Formal charge calculations are applicable to all types of molecules, including macromolecules like proteins and nucleic acids. These calculations provide insights into the electron distribution within these complex structures, aiding in our understanding of their biological functions.
Conclusion
Mastering the calculation of formal charge is a critical step in the journey of understanding molecular behavior and reactivity. This concept serves as a foundation for countless chemical theories and applications, making it an essential skill for chemists of all disciplines.


