Carbon's Bonding Secrets: Unveiled
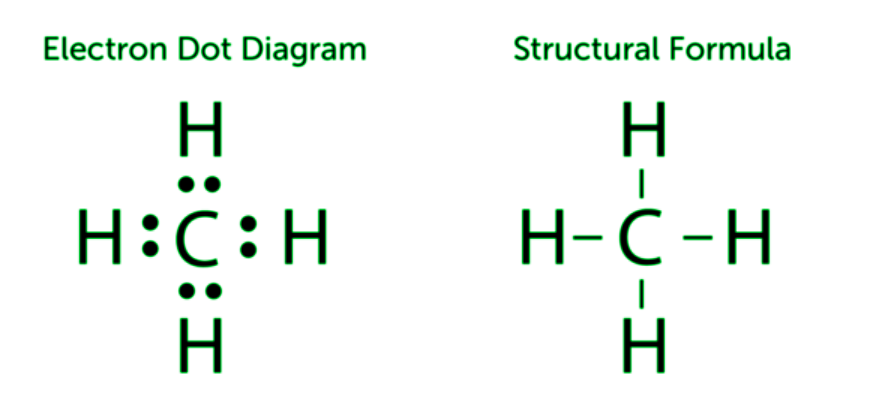
Diving into the intricate world of molecular interactions, we uncover the fascinating secrets behind carbon’s ability to form an astonishing array of compounds. This versatile element’s bonding versatility is a cornerstone of organic chemistry, shaping the very fabric of life as we know it. Let’s explore how carbon’s unique properties unlock a universe of chemical possibilities.
"Carbon's chemical diversity is a testament to its ability to form stable covalent bonds with itself and a wide range of other elements. This versatility is key to the complexity and richness of organic chemistry."
— Dr. Emma Johnson, Professor of Chemistry
Historical Perspective: Carbon’s Rise to Prominence
Carbon’s role in shaping human understanding of chemistry is a story that spans centuries. Early chemists, like Lavoisier and Priestley, laid the foundation by recognizing carbon as a distinct element. But it was the groundbreaking work of organic chemists in the 19th century that truly unveiled carbon’s bonding prowess.
The discovery of complex carbon-based compounds, from alcohols to carbohydrates, sparked a revolution. Chemists realized that carbon’s ability to form chains and rings opened a vast chemical landscape. This period saw the birth of structural theory, with scientists like Kekulé and Couper proposing groundbreaking models to explain carbon’s bonding behavior.
Carbon's bonding versatility was a pivotal factor in the emergence of organic chemistry as a distinct field of study, driving rapid advancements in our understanding of chemical structures and reactions.
The Four Bonding Types: A Technical Breakdown
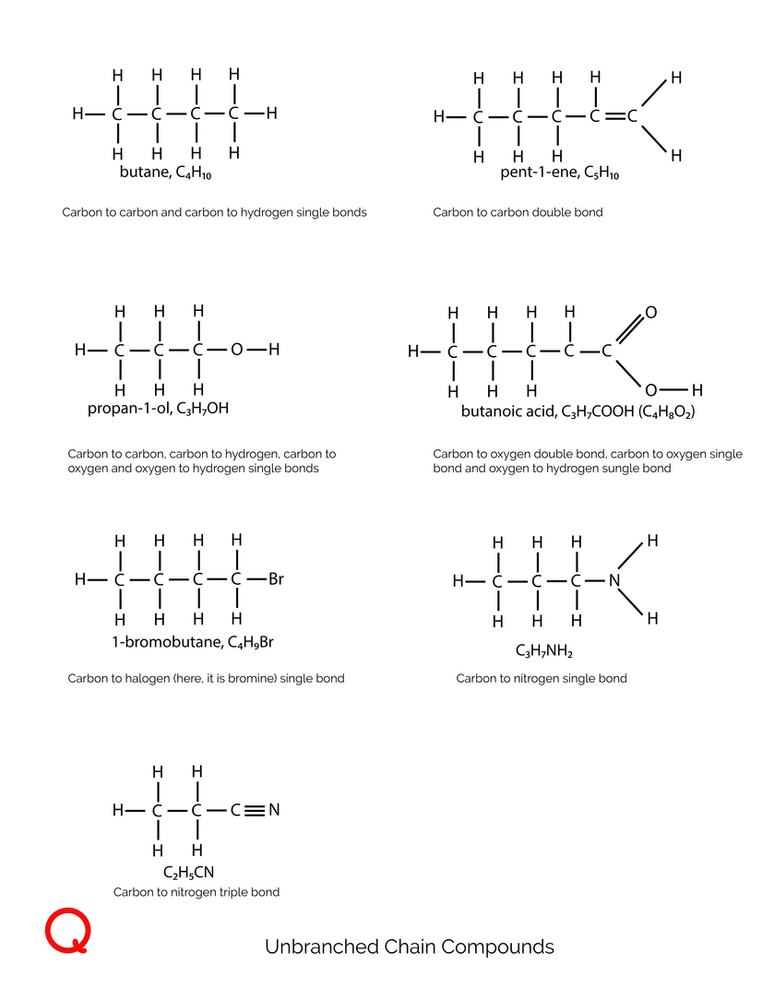
Carbon’s bonding secrets lie in its ability to form four distinct types of bonds:
Covalent Bonds: These are the cornerstone of carbon’s chemistry. Carbon atoms share electrons with each other or other elements, forming stable covalent bonds. This sharing allows carbon to build extensive networks, leading to complex molecules.
Ionic Bonds: While less common, carbon can also form ionic bonds with highly electronegative elements like oxygen or nitrogen. In these cases, carbon donates electrons, resulting in ionic compounds.
Metallic Bonds: In certain carbon-rich structures, like graphite, carbon atoms can form metallic bonds. These unique bonds give graphite its conductivity and other metallic-like properties.
Hydrogen Bonds: Carbon’s ability to form hydrogen bonds with neighboring molecules is crucial for the structure and function of biomolecules. These weak interactions stabilize the 3D shapes of proteins and DNA.
Unraveling the Hybridization Mystery
One of carbon’s most intriguing secrets lies in its ability to hybridize its orbitals. Through this process, carbon atoms can adjust their electron configurations to form different types of bonds. There are three main types of hybridization:
sp Hybridization: In this state, one s and one p orbital combine to form two equivalent sp hybrid orbitals. This arrangement allows carbon to form linear structures, as seen in carbon-carbon triple bonds.
sp2 Hybridization: Here, one s and two p orbitals hybridize, creating three sp2 orbitals. This geometry results in trigonal planar structures, often found in carbon-carbon double bonds.
sp3 Hybridization: The most common form, where one s and three p orbitals combine to create four equivalent sp3 orbitals. This leads to tetrahedral structures, as seen in carbon-carbon single bonds.
Understanding Hybridization Through an Example
- Consider ethene (C2H4), a simple alkene. In ethene, each carbon atom is sp2 hybridized.
- The two sp2 orbitals of each carbon atom overlap to form two carbon-carbon sigma (σ) bonds. These bonds are strong and stable.
- The remaining sp2 orbital on each carbon atom overlaps with the 1s orbital of a hydrogen atom, forming two carbon-hydrogen sigma (σ) bonds.
- Finally, the unhybridized 2p orbital on each carbon atom combines to form a pi (π) bond. This π bond is weaker and more reactive than the σ bonds.
Carbon’s Impact on Life: A Case Study
Carbon’s bonding versatility is nowhere more evident than in the realm of biochemistry. The intricate structures of biomolecules, from DNA to proteins, rely on carbon’s ability to form diverse bonds. Let’s explore this through the lens of amino acids, the building blocks of proteins:
Peptide Bonds: Amino acids link together through peptide bonds, formed by a carbonyl carbon of one amino acid and the amino nitrogen of another. This bond, a combination of covalent and ionic character, is crucial for protein structure.
Hydrogen Bonding: Within proteins, amino acids engage in extensive hydrogen bonding. This weak interaction stabilizes the 3D structure of proteins, influencing their functions.
Disulfide Bonds: In some proteins, cysteine residues can form disulfide bonds, a type of covalent bond. These bonds add stability and rigidity to protein structures.
Looking Ahead: Future Trends in Carbon Chemistry

As our understanding of carbon’s bonding secrets deepens, exciting avenues for exploration emerge:
Nanotechnology: Carbon’s ability to form stable, nanoscale structures, as seen in fullerenes and nanotubes, holds immense potential for innovative materials.
Green Chemistry: With a focus on sustainability, researchers are exploring carbon-based catalysts and processes that minimize environmental impact.
Biomimicry: Studying carbon’s role in biomolecules inspires new approaches to materials design, with potential applications in medicine and energy storage.
What makes carbon so unique in its bonding capabilities compared to other elements?
+Carbon's unique electronic configuration, with four valence electrons, allows it to form stable covalent bonds with itself and other elements. This versatility is unmatched by most other elements, leading to an incredibly diverse range of compounds.
How does carbon's ability to hybridize its orbitals impact its bonding behavior?
+Hybridization allows carbon to adjust its electron configuration, forming different types of bonds. This adaptability enables carbon to build diverse molecular structures, from linear chains to intricate rings.
What are some real-world applications of carbon's bonding versatility in materials science?
+Carbon's bonding secrets underpin the development of advanced materials like carbon fibers, graphene, and carbon nanotubes. These materials find applications in aerospace, electronics, and medicine, showcasing carbon's immense practical value.
Can you provide an example of how carbon's bonding influences the behavior of a specific molecule or compound?
+Take glucose, a simple sugar molecule. Carbon's ability to form both single and double bonds allows glucose to adopt a specific ring structure. This structural feature is crucial for glucose's role in energy metabolism, as it facilitates enzyme recognition and binding.
In conclusion, carbon’s bonding secrets are a testament to the intricate beauty of molecular interactions. Its ability to form diverse bonds and hybridize its orbitals opens a vast chemical landscape. From the foundations of organic chemistry to cutting-edge materials science, carbon’s versatility continues to shape our understanding of the world around us.


