The Mystery of Carbon's Bonding Capacity: Unveiled
Carbon, an element with a relatively simple atomic structure, possesses an extraordinary capacity to form an extensive range of chemical bonds, resulting in a vast array of compounds. This unique ability, often referred to as carbon’s “bonding capacity,” has long intrigued scientists and remains a cornerstone of modern chemistry. Understanding the intricacies of carbon bonding is not only fundamental to the study of chemistry but also pivotal in fields like material science, environmental science, and biotechnology.
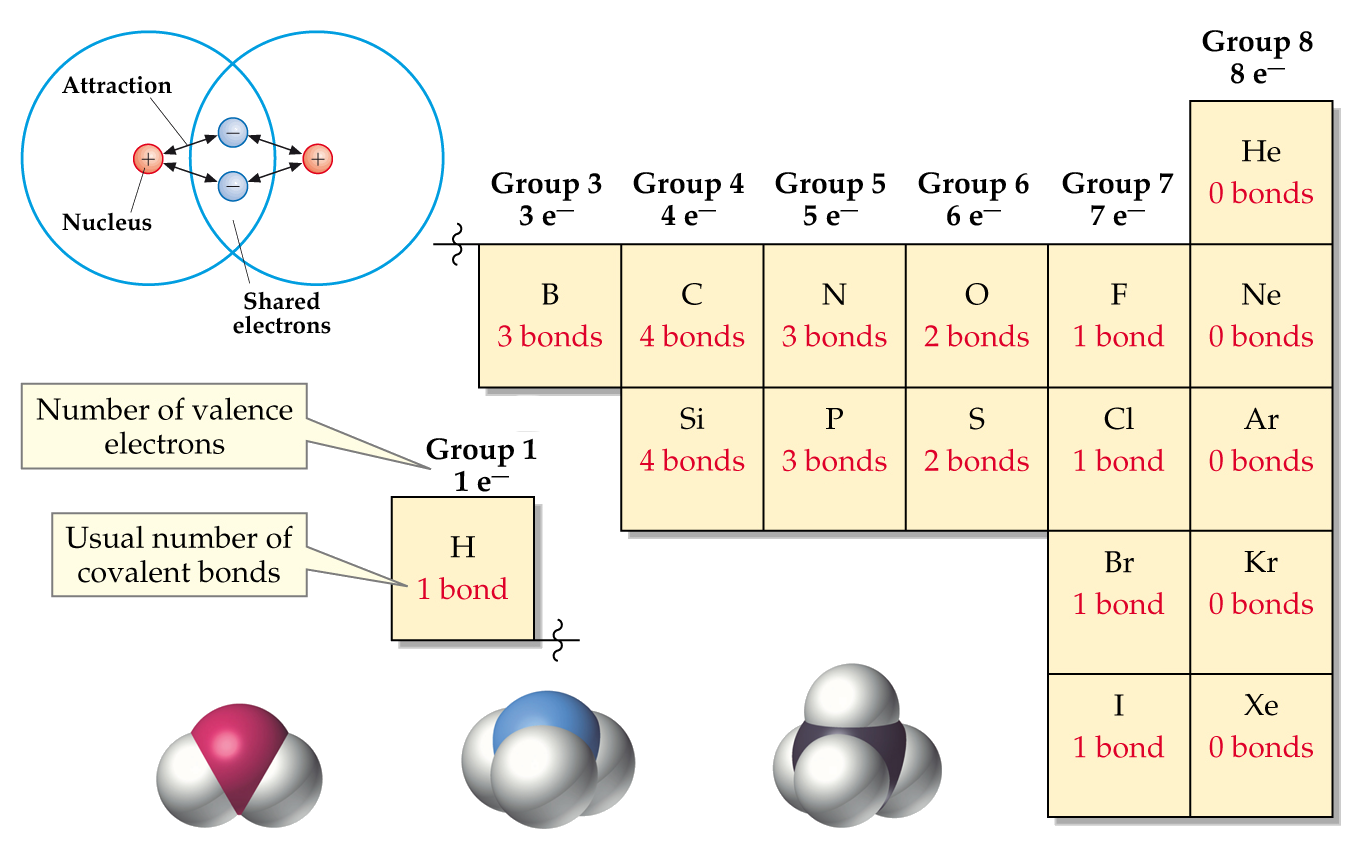
At the heart of carbon’s bonding versatility lies its electron configuration. With four valence electrons, carbon can either share these electrons to form covalent bonds or accept additional electrons to participate in ionic bonds. This dual capability allows carbon to form stable structures with a wide range of elements, leading to an extraordinary diversity of compounds.
One of the key factors contributing to carbon’s bonding capacity is its ability to form multiple bonds. Unlike many other elements, carbon can form double and triple bonds with other atoms, particularly with itself. This ability to create diverse bonding configurations significantly expands the possibilities for molecular structures.
For instance, carbon’s capacity to form double bonds with oxygen results in compounds like carbon dioxide (CO2), a greenhouse gas crucial to the Earth’s climate system. In contrast, carbon’s triple bonds with nitrogen give rise to compounds like cyanogen (C2N2), a highly reactive and toxic gas. These varied bonding patterns showcase the element’s remarkable versatility.
Furthermore, carbon’s bonding capacity is not limited to simple structures. Through the process of catenation, carbon atoms can bond to one another in long chains or complex networks, forming an array of organic compounds. This capacity to form extensive molecular networks is the basis for the immense diversity of organic life on Earth.
"Carbon's unique ability to form diverse bonds and complex structures is a testament to the complexity and beauty of the natural world. It's a cornerstone of chemistry and biology, and understanding its intricacies is key to unlocking the potential of many fields."
– Dr. Emma Young, Professor of Chemistry
The study of carbon bonding is not merely academic; it has profound real-world implications. For example, understanding carbon’s bonding capacity is crucial in fields like materials science, where researchers aim to develop new materials with specific properties. Carbon’s ability to form strong, stable bonds is also essential in environmental science, where the behavior of carbon-based compounds, such as hydrocarbons, is a key focus.
In the following sections, we will delve deeper into the various aspects of carbon bonding, exploring the different types of bonds carbon forms, the factors influencing its bonding capacity, and the implications of this unique property across various scientific and industrial fields.
We will also examine some of the challenges and opportunities associated with carbon’s bonding capacity, from its role in climate change to its potential in the development of new sustainable materials. This exploration will provide a comprehensive understanding of this fundamental aspect of chemistry and its far-reaching implications.



