Unveiling the Mystery: How Ions Form
Ions: The Building Blocks of Electrical Charge

Ions, often overlooked in their simplicity, play a pivotal role in the intricate dance of atomic interactions. Yet, their formation remains a fascinating enigma, a process that shapes the very fabric of our physical world. This journey into the heart of atomic chemistry will uncover the secrets of ion formation, shedding light on the underlying mechanisms that govern this fundamental phenomenon.
Ions, in essence, are atoms or molecules that have gained or lost electrons, resulting in a net electric charge. This charge can be either positive or negative, depending on the number of electrons acquired or lost. The process of ion formation, therefore, involves a delicate balance between the forces that hold an atom together and the external factors that disrupt this equilibrium.
The formation of ions is a multifaceted process, influenced by a myriad of factors. It is a complex interplay of atomic structure, chemical properties, and environmental conditions. At the heart of this process lies the concept of electron affinity and ionization energy, two key factors that determine an atom’s propensity to form ions.
Electron Affinity and Ionization Energy: The Dual Forces

Electron Affinity: Electron affinity refers to an atom’s ability to attract and bind with electrons. Atoms with high electron affinities readily accept electrons, leading to the formation of negative ions or anions. This affinity is influenced by factors such as atomic size, nuclear charge, and the electronic configuration of the atom.
Ionization Energy: In contrast, ionization energy represents the energy required to remove an electron from an atom. Atoms with low ionization energies readily lose electrons, resulting in the formation of positive ions or cations. This energy is determined by the strength of the atomic forces holding the electrons in place.
The delicate balance between electron affinity and ionization energy dictates the direction and extent of ion formation. Atoms with high electron affinities and low ionization energies are prime candidates for ion formation, readily accepting or donating electrons to form stable ionic compounds.
The Dance of Ion Formation
The process of ion formation can be visualized as a delicate dance, where atoms interact and exchange electrons to achieve a stable electronic configuration. This dance is governed by the principles of electrostatics and the desire of atoms to attain a state of minimal energy.
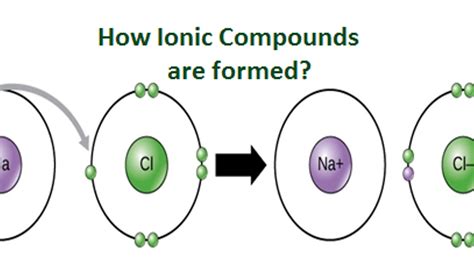
When two atoms interact, their electron clouds overlap, leading to the formation of chemical bonds. In the case of ionic bonding, one atom donates an electron to another, resulting in the formation of a positive ion (cation) and a negative ion (anion). This transfer of electrons is driven by the desire of atoms to attain a noble gas electronic configuration, a state of maximum stability.
The resulting ions, with their net electric charge, are then attracted to each other by electrostatic forces, forming an ionic compound. This compound, with its lattice-like structure, represents a stable arrangement where the positive and negative ions are held together by strong electrostatic attractions.
Factors Influencing Ion Formation
Several factors influence the propensity of an atom to form ions, shaping the intricate landscape of ion chemistry. These factors include:
Atomic Size: Smaller atoms tend to have higher ionization energies and lower electron affinities, making them less likely to form ions. In contrast, larger atoms, with their greater electron cloud size, have lower ionization energies and higher electron affinities, favoring ion formation.
Nuclear Charge: The positive charge of the nucleus, which holds the electrons in place, plays a crucial role in ion formation. Atoms with high nuclear charges tend to have higher ionization energies, making them less likely to form ions. However, this same nuclear charge also increases the electron affinity, enhancing the atom’s ability to accept electrons and form anions.
Electron Configuration: The electronic configuration of an atom, particularly the valence electrons, determines its propensity to form ions. Atoms with incomplete outer shells are more likely to form ions, either by accepting or donating electrons to complete their outer shell.
Environmental Conditions: The surrounding environment, including temperature, pressure, and the presence of other chemical species, can significantly influence ion formation. High temperatures and pressures, for instance, can increase the likelihood of ion formation by providing the necessary energy to overcome ionization barriers.
The Impact of Ions: A World of Applications

The formation of ions has far-reaching implications, shaping the very essence of our physical and chemical world. Ions are the building blocks of numerous chemical compounds, from simple salts to complex proteins. They are the key players in many chemical reactions, acting as catalysts, reactants, or products.
Ions also play a vital role in biological systems, influencing cellular processes and maintaining the delicate balance of the human body. They are essential for nerve function, muscle contraction, and the transport of nutrients and waste products.
In the realm of technology, ions are harnessed for a multitude of applications. From ion thrusters in space exploration to ion exchange resins in water treatment, the ability to control and manipulate ions has revolutionized various industries.
Unraveling the Mystery: A Journey Continues
The formation of ions, though unveiled, continues to be a subject of intense scientific exploration. As we delve deeper into the atomic realm, new questions and mysteries emerge, pushing the boundaries of our understanding.
The intricate interplay of forces, the delicate balance of electron affinities and ionization energies, and the myriad factors influencing ion formation present a complex and fascinating puzzle. Each discovery brings us closer to a comprehensive understanding of the atomic world, a world where ions play a pivotal role.
As we continue to unravel the mysteries of ion formation, we unlock the potential for innovative applications, from advanced materials to sustainable energy solutions. The journey into the heart of atomic chemistry is a never-ending quest, a pursuit of knowledge that drives us forward into the uncharted territories of scientific discovery.
What is the primary difference between electron affinity and ionization energy?
+Electron affinity refers to an atom’s ability to attract and bind with electrons, while ionization energy represents the energy required to remove an electron from an atom. Electron affinity is influenced by factors such as atomic size, nuclear charge, and electronic configuration, while ionization energy is determined by the strength of the atomic forces holding the electrons in place.
How do atomic size and nuclear charge influence ion formation?
+Atomic size and nuclear charge play a crucial role in ion formation. Smaller atoms tend to have higher ionization energies and lower electron affinities, making them less likely to form ions. Larger atoms, with their greater electron cloud size, have lower ionization energies and higher electron affinities, favoring ion formation. Similarly, atoms with high nuclear charges tend to have higher ionization energies, making them less likely to form ions, but their increased nuclear charge also enhances their electron affinity, making them more likely to accept electrons and form anions.
What are some real-world applications of ions?
+Ions have a wide range of applications in various fields. In chemistry, they are essential for the formation of numerous chemical compounds, from simple salts to complex proteins. In biology, ions play a crucial role in maintaining the balance of the human body, influencing cellular processes, nerve function, and muscle contraction. In technology, ions are harnessed for applications such as ion thrusters in space exploration and ion exchange resins in water treatment.
What are the key factors that determine the stability of an ionic compound?
+The stability of an ionic compound is determined by the strength of the electrostatic attractions between the positive and negative ions. This, in turn, is influenced by factors such as the charge of the ions (higher charges lead to stronger attractions) and the distance between the ions (closer distances lead to stronger attractions). The overall stability of an ionic compound is a delicate balance between these factors and the energy required to break the ionic bonds.


