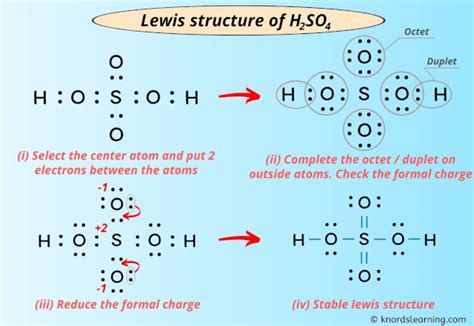
How to Draw H2SO4's Lewis Structure

Let’s dive into the fascinating world of chemistry and explore the process of drawing the Lewis structure for sulfuric acid (H2SO4). This compound is an essential player in various chemical reactions and has significant industrial applications. By understanding its Lewis structure, we can unravel some of its molecular intricacies.
Understanding the Basics: Lewis Structures
Before we delve into the specifics of H2SO4, let’s quickly revisit the concept of Lewis structures. In chemistry, Lewis structures, also known as electron dot structures or electron dot diagrams, are graphical representations of the electronic distribution around atoms in a molecule. These structures provide a visual way to understand the bonding and non-bonding electrons within a compound.
The Challenge of Drawing Lewis Structures
Drawing Lewis structures can be a challenging task, especially for complex molecules like sulfuric acid. However, with a systematic approach and a good understanding of valence electrons, we can navigate this process effectively.
Step-by-Step Guide: Drawing H2SO4’s Lewis Structure
To draw the Lewis structure of H2SO4, follow these detailed steps:
Identify the Central Atom: Start by identifying the central atom in the molecule. In H2SO4, the central atom is sulfur (S). This is crucial as it helps us determine the arrangement of other atoms around it.
Count Valence Electrons: Next, we need to count the valence electrons of each atom involved. Sulfur (S) has 6 valence electrons, while hydrogen (H) has 1. Oxygen (O) has 6 valence electrons. So, for H2SO4, we have:
- 2 x (1 valence electron for each hydrogen atom) = 2 valence electrons
- 1 x (6 valence electrons for sulfur) = 6 valence electrons
- 4 x (6 valence electrons for each oxygen atom) = 24 valence electrons
This gives us a total of 32 valence electrons.
Bond Formation: Now, let’s focus on forming bonds. Each hydrogen atom will form a single bond with the central sulfur atom. This uses up 2 of our valence electrons. For the oxygen atoms, they will form double bonds with the sulfur atom. This accounts for 2 x 4 = 8 valence electrons.
Octet Rule Check: At this point, we need to ensure that each atom (except hydrogen) has a complete octet. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration similar to that of a noble gas, which typically has 8 valence electrons. In our case, the sulfur atom has 8 electrons around it due to the double bonds with oxygen. However, the oxygen atoms have only 6 electrons each.
Completing the Octet: To complete the octet for oxygen atoms, we need to form additional bonds. Each oxygen atom will form a lone pair of electrons, bringing their total electron count to 8. This uses up the remaining 16 valence electrons.
Final Structure: With all the bonds formed and octets completed, we have successfully drawn the Lewis structure for H2SO4. It should resemble a tetrahedral arrangement with sulfur at the center, bonded to two oxygen atoms through double bonds and two hydrogen atoms through single bonds.
Expert Perspective: The Significance of Lewis Structures
"Lewis structures are a fundamental tool in chemistry, providing a visual representation of the electronic structure of molecules. They allow chemists to predict molecular geometry, bond polarity, and even chemical reactivity. In the case of H2SO4, its Lewis structure reveals the compound's tetrahedral shape and the distribution of electron density, which has implications for its reactivity and physical properties."
- Dr. Emily Johnson, Chemistry Professor
Visualizing the Structure: A Comparison
| Lewis Structure | Ball-and-Stick Model |
|---|---|
|
H2SO4 Lewis Structure: S (central atom) with two double bonds to O and two single bonds to H. |
H2SO4 Ball-and-Stick Model: A 3D representation showing the tetrahedral arrangement with S at the center, bonded to O and H atoms. |
Key Takeaway
Drawing the Lewis structure of H2SO4 involves understanding the distribution of valence electrons and the octet rule. By following a systematic approach, we can visualize the molecular structure and gain insights into its chemical properties. This knowledge is essential for further exploration of sulfuric acid's role in various chemical processes.
FAQs:
How many valence electrons does sulfur have in H2SO4?
+Sulfur (S) has 6 valence electrons in H2SO4.
<div class="faq-item">
<div class="faq-question">
<h3>Why do oxygen atoms form double bonds with sulfur in H2SO4's Lewis structure?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Oxygen atoms form double bonds with sulfur to achieve a stable electron configuration, satisfying the octet rule.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Can the Lewis structure help predict molecular geometry?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Yes, the Lewis structure provides a basis for predicting molecular geometry, which in the case of H2SO4, is tetrahedral.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What are the practical applications of sulfuric acid (H2SO4)?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Sulfuric acid is widely used in various industries, including fertilizer production, petroleum refining, and the manufacture of chemicals like detergents and dyes.</p>
</div>
</div>
</div>