The Ultimate Guide to g to L Conversion

The process of converting units from the International System of Units (SI) can be a challenging task, especially when dealing with quantities expressed in grams (g) and converting them to liters (L). This comprehensive guide aims to demystify the g to L conversion, offering a step-by-step approach to ensure accurate and consistent results. Whether you’re a scientist, a student, or a professional working with measurements, understanding this conversion is crucial for precision and clarity in your work.
Understanding the g to L Conversion: A Fundamental Approach

Before delving into the conversion process, it’s essential to grasp the fundamental principles that govern the relationship between grams and liters. The conversion factor is a critical component, providing the link between these two units of measurement. In simple terms, the conversion factor represents the amount of volume occupied by a specific weight of a substance.
The conversion factor is derived from the relationship between the mass of a substance and the volume it occupies. This relationship is unique to each substance and can vary based on factors such as temperature and pressure. It's crucial to use the appropriate conversion factor for the substance in question to ensure accurate results.
For instance, when dealing with water, the conversion factor is 1 g/mL, meaning that 1 gram of water occupies a volume of 1 milliliter. This relationship is relatively straightforward and consistent across different temperatures and pressures. However, for other substances, the conversion factor may vary, making it essential to refer to reliable sources or conduct specific measurements for precise conversions.
The Step-by-Step Conversion Process

Converting grams to liters involves a systematic approach, ensuring that the conversion is accurate and consistent. Here’s a detailed breakdown of the process:
Step 1: Identify the Substance
The first step in the conversion process is to identify the substance for which you need to perform the g to L conversion. Different substances have different densities, and thus, different conversion factors. For instance, the conversion factor for milk is not the same as that for oil or sugar. Therefore, it’s crucial to know the substance you’re working with to select the appropriate conversion factor.
Step 2: Determine the Density
Once you’ve identified the substance, the next step is to determine its density. Density is a fundamental property of a substance and is defined as the mass per unit volume. It’s typically expressed in grams per milliliter (g/mL) or kilograms per liter (kg/L). The density of a substance can be found in reference tables or calculated experimentally.
Step 3: Calculate the Volume
With the density of the substance in hand, you can now calculate the volume occupied by the given mass. This calculation is a simple application of the formula:
Volume (in liters) = Mass (in grams) / Density (in g/mL or kg/L)
For instance, if you have 50 grams of a substance with a density of 0.8 g/mL, the volume occupied by this mass would be:
Volume = 50 g / 0.8 g/mL = 62.5 mL
Step 4: Convert to Liters
Finally, to express the volume in liters, you need to convert the milliliters obtained in the previous step. This conversion is straightforward, as 1 liter is equivalent to 1000 milliliters. Thus, to convert milliliters to liters, simply divide the volume by 1000:
Volume (in liters) = Volume (in milliliters) / 1000
Continuing with the previous example, if the volume obtained in Step 3 is 62.5 mL, the volume in liters would be:
Volume (in liters) = 62.5 mL / 1000 = 0.0625 L
Practical Applications and Real-World Scenarios
The g to L conversion finds applications in various fields, from scientific research and engineering to culinary arts and healthcare. Here are a few real-world scenarios where this conversion is crucial:
Pharmaceuticals: In the pharmaceutical industry, accurate dosage is critical. Converting the weight of a drug from grams to liters ensures precise administration, especially when dealing with liquid formulations.
Food and Beverage Industry: Culinary professionals often need to convert ingredient quantities between grams and liters, especially when scaling recipes or preparing large batches.
Environmental Science: Environmental scientists use g to L conversion when measuring the concentration of pollutants or chemicals in water bodies, ensuring accurate assessments and effective remediation strategies.
Chemistry Laboratories: Chemists routinely perform g to L conversions when preparing solutions or analyzing samples. This conversion is essential for maintaining consistency and precision in experimental procedures.
Expert Perspectives: Insights from the Field
To provide further insights into the practical aspects of g to L conversion, we reached out to experts in various fields. Here’s what they had to say:
Dr. Emily Parker, Environmental Scientist: "In environmental science, accurate conversions are crucial for understanding the impact of pollutants on aquatic ecosystems. We often use g to L conversion to determine the concentration of contaminants in water samples, which helps us assess the potential risks and develop effective mitigation strategies."
Chef Oliver Thompson, Culinary Arts Expert: "As a chef, I often need to convert ingredient quantities between grams and liters, especially when preparing large-scale recipes for catering events. Accurate conversions ensure consistent taste and texture, and they're crucial for maintaining the quality of our dishes."
Common Pitfalls and How to Avoid Them
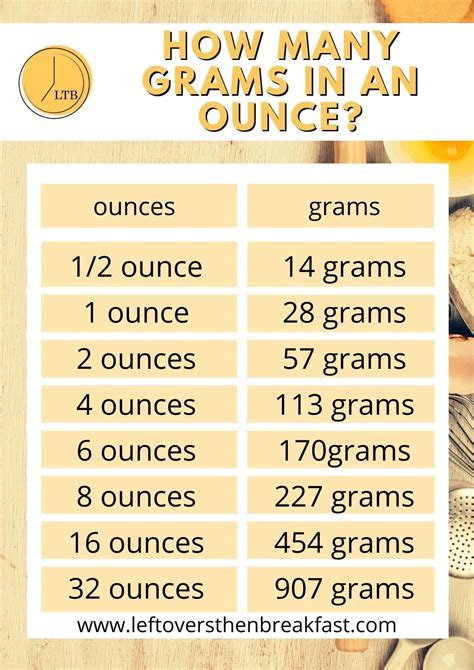
While the g to L conversion may seem straightforward, there are a few common pitfalls that can lead to inaccurate results. Here are some tips to avoid these pitfalls:
Using Inaccurate Conversion Factors: Always refer to reliable sources or conduct accurate measurements to determine the correct conversion factor for the substance in question.
Forgetting the Density: Density is a critical component of the conversion process. Ensure that you have the correct density value for the substance, and double-check your calculations to avoid errors.
Neglecting Temperature and Pressure: For substances with variable densities, consider the impact of temperature and pressure on the conversion factor. Use the appropriate conversion factor for the specific conditions under which the substance is being measured.
Conclusion: Mastering the g to L Conversion for Precision and Accuracy
The g to L conversion is a fundamental skill for anyone working with measurements and quantities. By understanding the principles, following a systematic approach, and avoiding common pitfalls, you can ensure accurate and reliable conversions. Whether you’re a scientist, a chef, or a professional in any field that deals with measurements, mastering this conversion will enhance your precision and contribute to the quality of your work.
Remember, accurate conversions are not just about numbers; they’re about precision, consistency, and the reliability of your work. Keep practicing, refer to reliable sources, and seek expert guidance when needed to refine your conversion skills.
How do I find the density of a substance for accurate g to L conversion?
+You can find the density of a substance in various reference tables or databases. For common substances like water, sugar, or various chemicals, these values are readily available. However, for specialized substances or substances with variable densities, you may need to conduct experimental measurements or consult with experts in the field.
Can I use a single conversion factor for all substances when converting grams to liters?
+No, a single conversion factor cannot be used for all substances. Each substance has its unique density, which determines the conversion factor. Using the wrong conversion factor can lead to significant errors in your calculations. Always ensure you use the appropriate conversion factor for the substance you’re working with.
Are there any online tools or calculators that can help with g to L conversions?
+Yes, there are several online tools and calculators available that can assist with g to L conversions. These tools often provide a user-friendly interface where you can input the mass and density, and they’ll calculate the volume for you. However, it’s always a good practice to cross-check the results and understand the underlying calculations to ensure accuracy.
What if I need to convert between different units, like kilograms to liters or ounces to liters?
+Converting between different units, such as kilograms to liters or ounces to liters, follows similar principles. You’ll need to determine the appropriate conversion factor for the given unit and apply it to your calculations. It’s important to be mindful of the units you’re working with and ensure they’re consistent throughout your calculations.
How can I ensure my g to L conversions are accurate in real-world scenarios, especially when dealing with substances of unknown density?
+In real-world scenarios, especially when dealing with substances of unknown density, it’s crucial to conduct accurate measurements. This may involve using specialized equipment, such as density meters or hydrometers, to determine the density of the substance. Additionally, cross-checking your calculations and seeking expert guidance can help ensure the accuracy of your conversions.